Article Text
Abstract
Background The aim of this study was to assess the technical success and procedural safety of the new Silk Vista device (SV) by evaluating the intraprocedural and periprocedural complication rate after its use in several institutions worldwide.
Methods The study involved a retrospective review of multicenter data regarding a consecutive series of patients with intracranial aneurysms, treated with the SV between September 2020 and January 2021. Clinical, intra/periprocedural and angiographic data, including approach, materials used, aneurysm size and location, device/s, technical details and initial angiographic aneurysm occlusion, were analyzed.
Results 60 aneurysms were treated with SV in 57 procedures. 66 devices were used, 3 removed and 63 implanted. The devices opened instantaneously in 60 out of 66 (91%) cases and complete wall apposition was achieved in 58 out of 63 (92%) devices implanted. In 4 out of 66 (6%) devices a partial opening of the distal end occurred, and in 5 (8%) devices incomplete apposition was reported. There were 3 (5%) intraprocedural thromboembolic events managed successfully with no permanent neurological morbidity, and 4 (7%) postprocedural events. There was no mortality in this study. The initial occlusion rates in the 60 aneurysms were as follows: O’Kelly–Marotta (OKM) A in 34 (57%) cases, OKM B in 15 (25%) cases, OKM C in 6 (10%) cases, and OKM D in 5 (8%) cases.
Conclusions Our study demonstrated that the use of the new flow diverter Silk Vista for the treatment of intracranial aneurysms is feasible and technically safe.
- aneurysm
- flow diverter
- technology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
The use of flow diverters (FDs) for the treatment of intracranial aneurysms has increased worldwide during the last decade, becoming the first-line approach in many centers.1 Although the main indication is wide-neck unruptured aneurysms arising from the paraclinoid or supraclinoid segments, advancements in FD technology developed in recent years have enabled a broadening of the indications, with treatment in smaller caliber vessels and in distal locations now feasible.2–5
One of the main changes to the procedural set-up has been the use of triaxial systems which occurred as a consequence of the fact that nearly all available FDs require the use of 0.027 inch (0.69) microcatheters for the deployment of a device designed for a 4 or 5 mm vessel.6–8 The complexity of the procedure is secondary to vessel tortuosity, where distal navigation of the microcatheter can be cumbersome, requiring robust coaxial constructions,9 10 or the opening/apposition of the braid may be affected. Increasing the number of devices and the procedural time have been related to intra/periprocedural complications.11 12
Although low profile stents and low-profile FDs have shown great benefits for the treatment of aneurysms located distal to the circle of Willis,12–14 the use of a lower profile FD for aneurysms arising more proximally has not been assessed.
The Silk Vista device (SV) (Balt, Montmorency, France) was launched in 2020, and is the only FD with all sizes compatible with a 0.021 inch inner diameter (ID) microcatheter for vessels ranging between 3.5 and 5 mm. The device has been redesigned from its predecessors Silk and Silk+, improving radiopacity and radial force.
We present a multicenter retrospective series of 60 consecutive aneurysms treated with the new SV FD. The aim of our study was to assess the technical success and the procedural safety of this new device by evaluating the intraprocedural and periprocedural complication rate after its use in several institutions worldwide.
Methods
The study involved a retrospective review of multicenter data regarding a consecutive series of patients with intracranial aneurysms, treated with the SV between September 2020 and January 2021 at 19 institutions worldwide. Institutional ethics committees approved this study. There were no exclusion criteria based on aneurysm location, type, size or clinical presentation.
Clinical, intra/periprocedural and angiographic data, including approach, materials used, aneurysm size and location, device/s, technical details and initial angiographic aneurysm occlusion, were analyzed.
All intra- and periprocedural events were evaluated. Clinical outcome was evaluated before treatment and at discharge using the modified Rankin scale (mRS). Minor events were considered if symptoms resolved within 7 days and major events if symptoms were present after 7 days. All events were evaluated during the hospitalization, focusing on the intra- and periprocedural period.
Aneurysm characteristics are summarized in table 1. Aneurysms were categorized as saccular, dissecting, fusiform or blister type in nature. The aneurysm size in the subgroup of saccular aneurysms (48 out of 60) were: 22 (46%) cases were small (<7 mm), 13 (27%) cases were medium (≥7 to <10 mm), nine (19%) cases were large (≥10 to <20 mm), one (2%) case was very large (≥20 to <25 mm), and three (6 %) cases were giant (≥25 mm).
Characteristics of aneurysms
Procedures
Antiplatelet therapy was mandatory before the procedure and was administered according to the local institutional protocols. An antiplatelet reactivity test was not mandatory. Antiplatelet therapy was continued after discharge per standard of care (table 2).
Clinical presentation and antiplatelet protocols
Available SV sizes range from 3.5 to 4.75 mm diameters with lengths between 15 and 30 mm. The device has been designed for vessels from 3.5 to 5 mm in size. All device sizes are delivered through a 0.021 inch catheter . The implant is made of 48 drawn filled tubing (DFT) wires (matching Nitinol with a platinum core into a single wire) which allows full radiopacity without additional platinum wires compared with its predecessors Silk or Silk+. It is 90% resheathable which is comparable to its smaller version, the Silk Vista Baby; however, the SV device does not have flared-ends and the radial force is five times more.
The stents were deployed through the following microcatheters: Headway-21 (MicroVention, Tustin, CA) in 45 cases, Rebar-18 (Medtronic Neurovascular, Irvine, CA) in 10 cases and Phenom-21 (Medtronic Neurovascular, Irvine, CA) in five cases. Intermediate catheters were used in 38 out of 57 (67%) procedures as follows: Navien 5/6F (Medtronic Neurovascular, Irvine, CA) in 13 cases, Sofia 6F/Sofia EX (MicroVention, Tustin, CA) in 13 cases, Cat 5 (Stryker Neurovascular, Fremont, CA) in four cases, Fargo Max (Balt, Montmorency, France) in three cases, Benchmark (Penumbra, Alameda, CA) in two cases and Vasco Mini (Balt, Montmorency, France) in one case. Nineteen procedures (33%) were performed without the use of an intermediate catheter.
The transfemoral approach was used in 48/57 (84%) of the cases, transradial in eight (14%) cases and transulnar in one (2%) case.
Initial occlusion rates were graded intraoperatively on the last angiographic run according to the O’Kelly–Marotta (OKM) grading scale for assessment of cerebral aneurysms treated by flow diversion (considering aneurysm filling as: A, total; B, sub-total; C, entry remnant; D, no filling).
Results
Sixty aneurysms were treated with SV in 57 procedures; 66 devices were used, three removed, and 63 implanted. The reasons for removal were: incorrect sizing (one case) and distal-end non-opening (two cases). On average 1.1 devices per aneurysm were implanted in this series.
In nine cases (15%) adjunctive coiling was performed using a jailing technique, seven (12%) cases were already coiled (in a previous procedure), and in 43 (73%) cases no coils were used (figure 1).
Paraophthalmic aneurysms treated with the Silk Vista device. (A) DSA, oblique view. A small unruptured paraophthalmic aneurysm. (B) Fluoroscopy images which demonstrate the radiopacity of the 4.5×20 mm braid despite the Nitinol design, and no flared-ends. (C) Post-implant DSA showing aneurysm filling with contrast stagnation (OKM A). (D) DSA, lateral view. A giant unruptured paraophthalmic aneurysm. The discrepancy of the proximal and distal arterial diameter was significant (1.4 mm). (E, F) A 4×30 mm device was deployed in combination with coils achieving a complete aneurysm occlusion (OKM D). DSA, digital subtraction angiography; OKM, O’Kelly–Marotta.
Among the 57 procedures, recapture and repositioning were performed in 18 cases. No microcatheter friction was noted.
The stented parent vessels had an average diameter of 4.2 mm (range 2.7–5.7 mm) proximally and 3.4 mm (range 2.2–4.93 mm) distally. The arterial proximal-distal discrepancy average was 0.8 mm (range 0–2.2 mm) (figure 2).
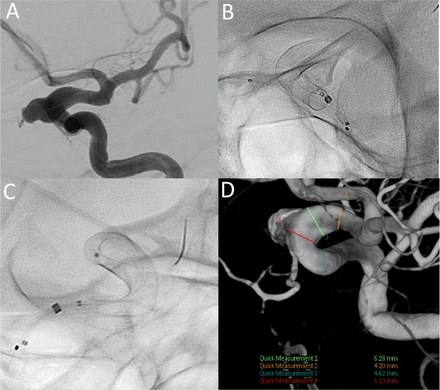
Sample case of an unruptured aneurysm treated with a single SV flow diverter and coils. (A) Dysplastic left ICA segment (6 mm) and paraophthalmic aneurysm. Note distal ICA measures 4.2 mm and proximal landing zone measures 4.5 mm. The dysplastic ophthalmic segment of the left ICA at the level of the aneurysm neck measures 6.1 mm. (B, C) A 4.75 mm x 25 mm SV device forshortened to 18.5 mm and fully opened to 6 mm through the dysplastic segment with excellent wall apposition. (D) Three dimensional reconstruction of the stented segment. ICA, internal carotid artery; SV, Silk Vista.
Braid opening
The devices opened instantaneously in 60 out 66 (91%) cases.
In four out of 66 (6%) devices a partial opening of the distal end of the SV occurred. In three of the four cases of distal-end partial opening, a significant discrepancy in the arterial diameter (>1.3 mm) was noted. Additionally, in two cases of distal-end non-opening, the location of the aneurysms was on the A1 segment resulting in braid oversizing. While two cases were managed intraoperatively, in the other two the operators decided to remove the device.
In one case (2%) the mid-portion of the stent remained partially constrained, in a 26 mm paraophthalmic aneurysm, which required a prior coil deployment to prevent microcatheter FD invagination. There was no restriction of flow and balloon angioplasty was used to successfully fully open the device.
The proximal end of the device opened fully in all cases, but in one case a slow opening was reported.
Wall apposition
A complete and full wall apposition was achieved in 58 out of 63 (92%) devices implanted.
In five (8%) devices, incomplete apposition was reported as follows: an incorrect distal-end wall apposition in two (3%) cases, one case related to a vessel angulation and another due to inappropriate sizing, an in-complete mid-stent apposition in one (2%) case (fully opened with a microwire internal massage), and an incomplete proximal-end apposition in two (3%) cases—in one case due to the vessel angulation and in the second case, a cervical internal carotid artery (ICA) dissecting aneurysm with a 5.3 mm vessel; a 4.75 mm device was selected and after implantation migrated into the aneurysm sac. Attempts were made to reposition the device; however, these proved unsuccessful and the patient was managed conservatively with no further complications or repeat hemorrhage.
In both cases of malapposition, secondary vessel angulation and stent positioning balloon angioplasty failed to resolve the malapposition. One case was initially managed conservatively but thrombosed 1 hour post-procedure despite adequate antiplatelet medication and resulted in acute neurological deterioration. The patient underwent a repeat procedure with implantation of a Leo+ stent (Balt, Montmorency, France) proximally to fully open the implanted SV, improving the clinical condition with no neurological sequelae (table 3, figure 3). In the second case, an Atlas stent (Stryker Neurovascular, Fremont, CA) was telescoped distally with no clinical sequelae.
Intra- and periprocedural complications
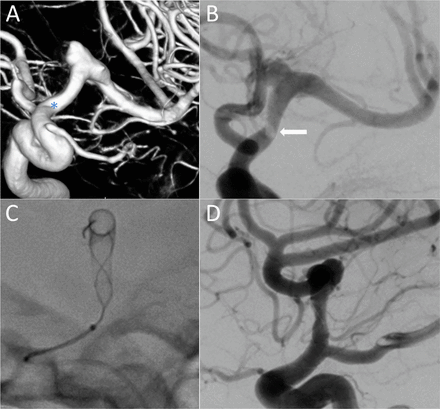
Left unruptured M1 dissecting/saccular aneurysm. (A) A 3.5×25 mm device was deployed from M1 to the supraclinoid ICA. The proximal landing zone was located into an arterial angulation (*), and the device did not appose fully to the ICA wall. After unsuccessful attempts of repositioning with balloon angioplasty, it was managed conservatively. (B) One hour after the procedure, the patient developed right hemiparesis and a DSA showed stent thrombosis (arrow) secondary to the proximal stent malapposition. (C) A bolus of tirofiban was administered and a Leo+ stent was proximally telescoped allowing a fully wall apposition. (D) Both stents remained opened, fully patent with no flow compromise. DSA, digital subtraction angiography; ICA, internal carotid artery.
The initial occlusion rate in the 60 aneurysms were as follows: OKM A in 34 (57%) cases, OKM B in 15 (25%) cases, OKM C in six (10%) cases, and OKM D in five (8%) cases.
Intraprocedural events
There were three (5 %) intraprocedural thromboembolic events, all of them related to stent malapposition as described above, with no permanent neurological morbidity (table 3).
There were no aneurysm ruptures or dissections and no mortality as a result of the procedure.
Periprocedural events
Four (7%) events were documented post-procedure as follows: an immediate post-procedural stent thrombosis (table 3, figure 3) resolved with a telescoped Leo+ stent (no clinical consequences but an ischemic stroke in the left basal ganglia on 24 hours CT was reported); one external ventricular drain tract hemorrhage post-procedure in a case of sub-arachnoid hemorrhage (SAH) (stable after eptifibatide shut off, resolved at time of discharge from hospital with no new focal neurological deficits); one hemi-pontine stroke in a case of symptomatic dissecting mid-basilar aneurysm; and one groin superficial hematoma with no associated pseudoaneurysm.
There was no mortality in this study.
The median length of hospital stay was 3.4 days (range 1–23 days). The mRS score at discharge from the hospital did not change from the admission mRS score except in three patients: two patients with SAH were discharged with mRS 2 after hospitalization; and one patient presenting with a symptomatic dissecting midbasilar aneurysm was discharged with mRS 2, with a favorable evolution at 1 month (mRS 1).
Discussion
Over the past decade, flow diversion has increasingly been replacing conventional techniques as the first-line endovascular treatment for many types of intracranial aneurysm.1
Since the CE mark of the Silk FD device in 2008, three generations of the device have been launched in the last 10 years. Some technical modifications have improved the visibility and the radial force of the implant.15 16
The first generation Silk was constructed from 48 braided wires—44 Nitinol wires and four platinum wires and four platinum coils. The original Silk device was characterized by its flexibility and ability to adapt to the arterial anatomy; however, this limited the pushability and trackability in tortuous vessels, with poor opening on deployment due to lower radial force.
The second generation device (CE mark in 2012), the so-called Silk+, was made of 48 wires with higher radial force, eight platinum wires and four platinum coils, designed to improve visibility and flared-ends to optimize wall apposition. It was compatible with 0.021 inch and 0.025 inch microcatheters and could be resheathed up to 90%.
Lubicz et al 15 reported a single center experience with the use of both the Silk and Silk+ in 58 patients with 70 aneurysms: 32 patients were treated with the Silk and 26 with the Silk+. Clinical periprocedural complications occurred in 15%, the overall permanent neurologic morbidity rate was 5.5%, and there was no procedure-related mortality. Embolization was successful in 54 patients (93%), and failure occurred in four patients (7%).
In the DIVERSION16 prospective cohort study, 122 Silk devices were deployed in 118 patients. The authors reported three thromboembolic events, six incomplete deployments, one dissection and two complications at the puncture site.
In our series, 63 devices were deployed in 57 patients, with three thromboembolic complications and three devices removed. No dissections and no procedure-related mortality were reported. All the thromboembolic complications were related to stent malapposition and this was at least partly due to the underlying vascular anatomy and the landing sites as well as the discrepancy between the diameters of the different vessel segments where the device would be deployed.
Although the safety and efficacy of the first two generations of the device have been demonstrated in large clinical series, a principal limitation of these early generation devices, as compared with cobalt-chromium FDs, was the radiopacity and radial force.5
Although all FD devices follow the same hemodynamic concept, the companies embarked on the development and manufacturing of a variety of braids with different properties during the last years. We could divide the FDs into two representative groups: cobalt-chromium and Nitinol devices (table 4). While cobalt-chromium implants have the advantage of a higher radial force and greater distal anchor stability during deployment, the Nitinol FDs are more trackable and require lower profile microcatheters (figure 4). Although the stent design may have a theoretical impact, depending on anatomical tortuosity of the parent artery or aneurysm location, no significant clinical differences between FDs have been reported so far.
Paraophthalmic aneurysm, lateral view. (A) A Headway-21 microcatheter was intentionally navigated into the aneurysm lumen for distal advance into the supraclinoid ICA due to the anatomical location and the wide neck. An SV device was tracked without friction and opened by push-pull technique (B–E). Once the distal third of the SV device was opened and anchored, the microcatheter was gently pulled back and centered into midline for deployment. ICA, internal carotid artery; SV, Silk Vista.
Overview of the characteristic of the current flow diverters
Our intra- and periprocedural results should be compared with the more recent literature evidence on FDs.
In 2020, Rice et al 17 published the results of the large prospective study using the Pipeline Flex embolization device with Shield technology for the treatment of 204 aneurysms. The average of the devices was 1.1, the same as for our series. Four out of 252 devices were removed and adjunctive coils were added in 18.6%. Complete apposition was achieved in 93.1% of cases. Complete occlusion of the aneurysm was achieved in 1% of the cases. Periprocedural stroke occurred in 6.4% during the first 30 days.
In our series wall apposition was achieved in 92%, complete aneurysm occlusion in 8% and thromboembolic events in 5%.
Also in 2020, Maus et al 18 published a case series using the new Surpass Evolve. Like the Pipeline Shield, devices were deployed using a triaxial system with a 0.027 inch microcatheter. Authors reported 46 aneurysms treated with the device, but the average was 1.2, slightly higher than in our series. Adjunctive coiling was performed in 37% of the cases; however, the authors did not report the immediate OKM occlusion rate. Intraprocedural events occurred in 2% (one patient suffering a stent thrombosis managed endovascularly without sequelae) and mortality occurred in 2% (in a patient who presented with an SAH). They reported two unsuccessful deployments, related to tortuous vessel anatomy.
In our series, all cases were consecutive and there were no unsuccessful deployments.
In 2019, Pierot et al 19 presented the results of the prospective SAFE study, using FRED and FRED Jr devices, for the treatment of 103 aneurysms. Despite being made of Nitinol, FRED requires a 0.027 inch microcatheter for delivery, while FRED Jr, designed for distal vessels ranging from 2.5 to 3 mm, is designed for deployment through a 0.021 inch microcatheter. Treatment was successfully performed in 95% of cases. Thromboembolic complications occurred in 6.8%, three during the procedure or immediately after (within 6 hours) and four after the procedure (1.4 and 7 days with a further event at 14 months post-procedure). Intraoperative rupture occurred in two of 103 patients (1.9%).
The SV is the only FD made with 48 wires which is 0.021 inch compatible and available for vessels up to 5 mm. Recently another Nitinol FD with the same 0.021 inch profile has been launched, the P64 MW HPC; however, clinical experience for this device is limited at the moment.20
In 2018 the third generation device, the SVB, received CE approval for the treatment of aneurysms located in small or distal vessels (1.5–3.5 mm). The device used DFT technology and allowed full radio-opacity of the device. The SVB is constructed from 48 DFT wires and is the first FD that can be delivered through a 0.017 inch microcatheter.3 4
Recently, the periprocedural outcomes of SVB in a series of 41 patients with 43 small aneurysms (mean 9.5 mm) at and beyond to the circle of Willis were assessed.5 The intraoperative complete occlusion rate was 18.6%. There were five cases of intraprocedural complications with no clinical consequence. Despite the distal location, procedures without intermediate catheters/triaxial systems were performed in 20 out of 41 procedures.
The SV can be considered to be a larger version of the SVB and is designed for vessels with a diameter between 3.5 and 5 mm and compatible with a 0.021 inch microcatheter. The design utilizes the same DFT technology as seen in the SVB and is constructed from 48 DFT wires, but it does not have flared-ends. The radial force of the SV is considerably higher than that of the SVB with approximately five times greater radial force than the SVB. The flared-ends concept was introduced in 2007 with the Leo+ stents and continued with Silk, Silk+ and SVB, with the intention to prevent a potential device migration. In the case of SV the design is purely tubular and may help with achieving a more precise wall apposition.
FD malapposition is associated with an increased risk of stroke-related complications.10 11 In our study we identified wall malapposition in 8% of cases with affected segments at the proximal, mid and distal ends of the device. The improved visibility enabled by the DFT technology allowed this to be detected and would have gone unnoticed with the previous generation Silk or Silk+ devices.
Most of the FDs used in proximal locations remain compatible only with 0.027 inch ID microcatheters6–8 (table 4). While in distal locations there is a clear benefit to the use of lower profile stents and delivery catheters, the potential benefit of a lower profile 0.021 inch compatible device for vessels of 4 or 5 mm remains unclear. Beyond the evident benefits in terms of navigation through tortuous anatomies, it is our opinion that an 0.021 inch compatible FD could have several potential benefits over the more conventional 0.027 inch systems. One hypothetical benefit could be lowering the friction between the catheters, a source of polymer embolism. The excessive manipulation of tight-fitting devices in coaxial, triaxial and quadriaxial catheterization techniques within tortuous vessels, use of larger diameter devices, multiple or difficult catheterization through previously placed devices, repeat interventions, and the presence of calcific atherosclerotic debris may ‘scrape’ the polymer coating layer or at times even the base coat layer off the device.21–23
Other potential advantages could be the extra space within the internal lumen of the guiding catheter that would allow for improved contrast injections and hence improved angiography, better detection of intra-aneurysmal stasis, earlier detection of thrombus and distal complications, as well as more effective flushing which in turn may reduce the risk of thromboembolic complications. Similarly, in the case of telescoping, the navigation of lower profile ID microcatheters through a previously implanted device could reduce the risk of dislodging the initial device.
Microcatheter size is an important factor to consider when attempting to maneuver beyond the distal lip of an aneurysm. Different techniques have been proposed for advancing a 0.027 inch distal to an aneurysm for FD deployment.9 10 24 The inability to navigate beyond challenging segments can result in the need to abandon the preferred treatment approach or change the components of the coaxial system. There has been a paradigm shift in the design and approach to catheter support systems for cases of FD from a classic biaxial set-up to a more robust triaxial system.9 10 Despite the safe profile, concern for catheter-induced arterial injury and proximal tortuosity limits the performance applicability of distal intermediate catheters . In our series, 34% of the procedures were performed without the use of intermediate catheters, highlighting the trackability of the SV device (figure 4). Similarly, the requirement for extra delivery and access catheters also raises the cost of these procedures.
In braided stent FDs, the radial force is maximal in the center under nominal diameter, that is, the FD is not under constraint. The radial force drops rapidly toward both ends of the FD25 if the FD is under constraint when placed in an artery with a vessel diameter smaller than the nominal diameter. In our series, in three cases of sub-optimal opening of the distal-end of the device there was a significant arterial discrepancy and two of the cases occurred in a distal segment (A1) after braid oversizing. When a significant arterial discrepancy is present, the oversizing effect of a single device may be minimized by placing two telescoped devices of different sizes.
Physician experience with a device has been related to the risk of complications, highlighting the need for a learning curve.26 Despite the SV being a relatively new device, in this study the average number of devices implanted was 1.1 and intraprocedural events occurred in 5%, suggesting that an extensive learning curve is not required and the lessons of the past and experience with FDs in general is sufficient for experienced operators.
Our study has limitations including the outcomes of a retrospective multicenter experience that is not randomized, which leads to selection bias. This is a small sample size and long-term clinical outcomes are necessary for evaluating long-term safety and efficacy. Angiographic findings, such as evaluation of in-stent stenosis27 or delayed parent artery occlusions,28 need to be compared with previous generations. The sample did not include a significant number of giant aneurysms, where complex maneuvers are required, including telescoping devices in tortuous anatomies. We did not perform a post-procedural diffusion weighted imaging, which could be helpful to evaluate the potential emboli due to catheter frictions.
Our results appear promising, but larger series with longer-term follow-ups are needed to corroborate the effectiveness of this treatment method and its superiority to other devices or techniques.
Conclusion
Our study demonstrated that the use of the new FD Silk Vista for the treatment of intracranial aneurysms is feasible and technically safe.
Ethics statements
Patient consent for publication
Ethics approval
Institutional Review Board. CEIm Área de Salud Valladolid Este. PI 21–2169.
References
Footnotes
Twitter @Doctorgaldamez, @VladoKZg, @neurofox, @drschuller, @jdavidguio, @pnavia
Contributors MMG: study concept, literature review, acquisition of data, draft and review of the manuscript; PB: critical review. All authors have contributed to the authorship, and final review of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests MMG is proctor and consultant for Balt, Medtronic and Stryker. PG is consultant for Phenox, Balt and Cerenovus. JGF is consultant for Medtronic and Balt. MSA is consultant for Medtronic and Balt. PN is consultant and proctor for Balt, Stryker and Penumbra. The rest of the co-authors have not declared any conflict of interesting regarding this manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.