Article Text
Abstract
Background The treatment of acute ischemic stroke is traditionally centered on time criteria, although recent evidence suggests that physiologic neuroimaging may be useful. In a multicenter study we evaluated the use of CT perfusion, regardless of time from symptom onset, in patients selected for intra-arterial treatment of ischemic stroke.
Methods Three medical centers retrospectively assessed stroke patients with a National Institute of Health Stroke Scale of ≥8, regardless of time from symptom onset. CT perfusion maps were qualitatively assessed. Patients with defined salvageable penumbra underwent intra-arterial revascularization of their occlusion. Functional outcome using the modified Rankin Score (mRS) was recorded.
Results Two hundred and forty-seven patients were selected to undergo intra-arterial treatment based on CT perfusion imaging. The median time from symptom onset to procedure was 6 h. Patients were divided into two groups for analysis: ≤8 h and >8 h from symptom onset to endovascular procedure. We found no difference in functional outcome between the two groups (42.8% and 41.9% achieved 90-day mRS ≤ 2, respectively (p=1.0), and 54.9% vs 55.4% (p=1.0) achieved 90-day mRS ≤ 3, respectively). Overall, 48 patients (19.4%) had hemorrhages, of which 20 (8.0%) were symptomatic, with no difference between the groups (p=1.0).
Conclusions In a multicenter study, we demonstrated similar rates of good functional outcome and intracranial hemorrhage in patients with ischemic stroke when endovascular treatment was performed based on CT perfusion selection rather than time-guided selection. Our findings suggest that physiologic imaging-guided patient selection rather than time for endovascular reperfusion in ischemic stroke may be effective and safe.
- Stroke
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Background
The primary treatment guidelines of acute ischemic stroke are based on time-guided criteria rather than penumbral considerations. For nearly two decades studies have confirmed that intravenous tissue plasminogen activator (tPA) is safe and effective if given within the first 3 h of stroke symptom onset and subsequent studies have demonstrated clinical efficacy up to 4.5 h.1–4 In fact, further studies have shown that intra-arterial thrombolysis up to 6 h and mechanical thrombectomy up to 8 h from symptom onset with successful vessel recanalization is associated with improved patient outcomes.5–10
Despite the introduction of thrombolytic agents and novel endovascular approaches to acute ischemic stroke, patients often do not derive the maximum benefit from such therapies due to rigid time criteria that guide therapeutic utilization. However, recent applications of physiologic imaging including CT perfusion (CTP) and diffusion-weighted MRI aid in assessing regional blood flow, vascular autoregulation and neuronal integrity.11–13 Subsequent studies suggest that physiologic penumbral imaging patient selection for endovascular treatment is a viable means of extending time-guided selection.14–19
The implication of physiologic imaging criteria for patient selection in acute ischemic stroke is the capability of providing more patient-specific treatment options, particularly in large vessel obstructions and when patients are beyond traditional time-guided therapy recommendations.20 ,21In a multicenter study, we aimed to demonstrate that CTP imaging in the context of the patients clinical assessment can be an effective and safe method of selecting patients for neuroendovascular treatment.
Materials and methods
A collaborative retrospective study was performed at three medical centers (Medical University of South Carolina, University of Florida and the Swedish Medical Center in Denver Colorado), assessing the use of CTP-guided endovascular treatment of acute ischemic stroke. All institutions are comprehensive stroke centers that function as tertiary referral centers for regional multicenter stroke networks. Institutional review board approval was granted separately at each participating site. Chart reviews were performed at the three institutions and a central data sheet was used to document clinical, angiographic and follow-up outcomes.
The patient characteristics documented were age, gender, baseline National Institute of Health Stroke Scale (NIHSS) score at presentation, time from symptom onset to the start of endovascular procedure, vessel(s) occluded, concomitant administration of intravenous tPA, thrombolysis in cerebral infarction (TICI) scores, procedural complications (including symptomatic and asymptomatic hemorrhage) and modified Rankin Score (mRS) at 90 days or closest follow-up period to 90 days. mRS data were obtained from the neurology clinic record or, in cases where not specifically defined, it was extrapolated from the clinical record. If the medical record did not contain outcome information, an independent neurologist contacted the patient or family to determine the functional status of the patient in order to determine the mRS. Neurointerventional radiologists or neurosurgeons assessed radiologic and angiographic images to document the location of the vascular occlusion, recanalization time, TICI flow after the procedure, procedural complications and the presence of intracerebral hemorrhage after or during the procedure.
Patient selection
All stroke patients presenting with NIHSS ≥8, irrespective of time of symptom onset, were considered candidates for endovascular therapy. All patients underwent non-contrast CT, CT angiography (CTA) and CTP on admission to the emergency department. Admission non-contrast CT imaging was used to determine the presence and extent of gross infarction and to exclude underlying pathology such as mass or intracranial hemorrhage (ICH). CTA was used to identify the location of the vascular occlusion. CTP was analyzed to identify the presence and extent of penumbra relative to the core area of infarction. The primary use of CTP in the posterior circulation was to ensure that there was not complete brainstem/pontine infarction. Given the limitations in imaging the posterior circulation, we used CTP as an adjunctive scan. The primary endpoint of this multicenter study was good functional outcome as defined by 90-day mRS ≤ 2. Secondary efficacy endpoints included mRS ≤ 3 and recanalization by TICI ≥2b. The primary safety endpoints were symptomatic intracranial hemorrhage (sICH) within 36 h and mortality at 90 days.
CT perfusion imaging techniques
CT imaging was performed according to the individual standardized institutional protocol. All CTP scans were performed using a Siemens 64- or 16-slice scanner with a 50 ml bolus of Omnipaque 350 (GE Healthcare). Perfusion acquisition for the 64-slice Siemens took 49.44 s to acquire a total of 448 images in 32 scans, centered from the basal ganglia to the vertex with approximately 10 cm of coverage area, while the 16-slice scanner took 40 s to acquire 106 images covering 4 cm centered at the basal ganglia. Perfusion datasets were post-processed on a GE Advantage Windows Workstation (General Electric; Milwaukee, Wisconsin, USA), a Siemens Leonardo Workstation (Siemens Medical, Germany), or both. CTA was subsequently performed with an 80 ml bolus injection of contrast followed by a saline chase. Two-dimensional 1 cm thick slab reformations were created in the axial, sagittal and coronal projections. These data were used to localize major vessel occlusion.
Data processing
CTP post-processing on the GE workstation was performed by a radiology resident or by an experienced CT technologist yielding mean transit time (MTT), cerebral blood volume (CBV) and cerebral blood flow (CBF) maps. The CTP post-processing on the Siemens workstation was performed by an experienced CT technologist. MTT, CBV and CBF maps were reconstructed from the source images. The arterial input and venous outflow curves were analyzed to ensure complete data sets. All CTP interpretations for treatment were made by the treating neurointerventionalist based on qualitative interpretation of the perfusion maps. MTT and CBF maps were analyzed to define the area of brain at risk as delineated by at least two color band differences on the six-spectrum rainbow scale from the surrounding unaffected brain. CBV was evaluated to delineate the core region of infarction as the area with depressed CBV at least two color band differences on the six-spectrum rainbow scale from the surrounding unaffected brain. Patients with one-third or more of the MCA territory infarcted or with ≤50% penumbra were not considered candidates for endovascular treatment unless an eloquent area was at risk.
Endovascular treatment
All cases were performed under general anesthesia. All patients received intra-arterial heparin consisting of a bolus of 1500 units followed by a bolus of 500 units every hour thereafter. The primary method of treatment in most cases was mechanical aspiration with the Penumbra aspiration system. The approach that evolved over the first 10 cases was to use the largest caliber aspiration catheter that the occluded vessel would accommodate. Intra-arterial thrombolysis with tPA was used adjunctively at low doses ranging from 10 to 20 mg (administered in 3–5 mg aliquots) during the procedure if aspiration was not immediately effective. In patients who presented within the 0–3 or 3–4.5 h time windows, intravenous tPA was administered to eligible patients before going to the angiography suite. Intra-arterial abciximab was administered if acute intraprocedural thrombus formation was felt to be present. If a permanent stent was used either to recanalize the thrombosed vessel or to treat a proximal stenosis, the patient was given a bolus of a weight-based loading dose of abciximab and then 325 mg aspirin and 600 mg clopidogrel were administered immediately following the procedure. All patients were managed postoperatively in the neurosurgical intensive care unit for at least the first 24 h after the endovascular procedure to ensure strict blood pressure monitoring and control.
Data analysis
Baseline demographic, radiographic and outcomes data were analyzed using descriptive statistics including means, standard deviations, frequencies, percentages and medians. Comparisons between time-to-treatment groups were made using two-sided t tests for continuous measures, χ2 tests for categorical measures and Mann–Whitney U tests for non-parametric measures (medians). No adjustments were made for multiple comparisons, and p values of <0.05 were considered significant.
Results
Two hundred and forty-seven patients underwent endovascular treatment, with 47% receiving intravenous tPA prior to intra-arterial therapy. Demographic data, mean NIHSS score at presentation, location of vascular occlusion and the percent receiving bridging intravenous tPA therapy are shown in table 1.
Patient baseline characteristics for study group
The average time from the last point the patient was seen normal to groin vascular access was 8.2 h (median 6.0 h, range 1.5–77.4 h). Recanalization rates of TICI 2b or 3 were 75%. There were 28 (11.2%) procedural-related complications including vessel perforation and vessel dissection. Overall, 48 patients (19.4%) had bleeding complications including subarachnoid hemorrhage, parenchymal hemorrhage and intraventricular hemorrhage; 20 (8.0%) of these patients had sICH.
Patients were initially selected for endovascular procedures based on CTP criteria and then analyzed based on time from symptom onset to procedure. Patients were divided into two groups: ≤8 h and >8 h from symptom onset to neurointerventional procedure. Eight hours was chosen since the mean time to treatment was 8.2 h, which corresponds with the 8 h time criteria used for treatment in mechanical device trials.9 ,22 ,23 There were no significant differences in age, race or gender between the groups (table 2). The mean baseline NIHSS scores were 18.7 and 17.2 (p=0.069) for the two groups, respectively. For the two time periods, 42.8% and 41.9% achieved 90-day mRS ≤ 2, respectively (p=1.0), and 54.9% and 55.4% (p=1.0) achieved 90-day mRS ≤ 3, respectively (table 2). Additionally, the percent mortality, as measured by an mRS of 6 at 90-day follow-up, was 24.9% vs 20.3% (p=0.5) and the rate of ICH was 19.7% vs 18.9% (p=1.0) for the two groups, respectively (table 2).
Comparison in treatment and outcomes between patients treated ≤8 h and those treated >8 h from symptom onset
When recanalization was assessed in the two groups, 71.7% achieved TICI 2b or 3 in the ≤8 h group and 81.1% in the >8 h time group (p=0.151). The location of vessel occlusion was compared in the two groups. Anterior circulation occlusions comprised 92.4% and 83.6%, respectively, in the ≤8 h and >8 h time groups (p=0.062). When occlusions of the middle cerebral artery (MCA) were isolated, rates in the ≤8 h group were 69.6% compared with 67.1% in the >8 h group (p=0.763, table 2).
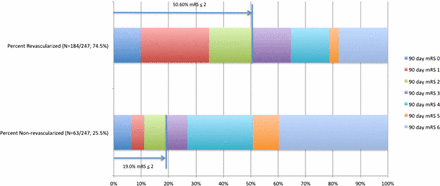
When the effect of recanalization on 90-day functional outcome was assessed, 50.6% of those who were recanalized achieved mRS ≤ 2 compared with 19.0% of those who were not recanalized. However, when recanalization rates and worse functional outcome (mRS 4–6) were compared, 73.0% of those who were not recanalized achieved mRS 4–6 compared with 35.3% of patients who were recanalized (figure 1).
Modified Rankin scores (mRS) at 90 days for patients who achieved recanalization (above) and those who did not achieve recanalization (below). Of those who were recanalized, 50.6% achieved mRS ≤ 2 at 90 days compared with 19.0% of those who were non-recanalized.
The administration of intravenous tPA did not significantly affect the number of patients experiencing a good clinical outcome (45% of those treated with intravenous tPA achieved mRS 0–2 compared with 41% of those not treated with tPA, p=0.54).
Discussion
In a multicenter study, we have demonstrated similar rates of good functional outcome and ICH in patients with acute ischemic stroke when endovascular treatment was performed based on CTP selection rather than time-guided selection. These findings suggest that endovascular reperfusion in ischemic stroke may be safe and effective when based on patient-specific data from physiologic imaging rather than rigid time-based criteria. The decision to treat patients with acute ischemic stroke beyond currently recommended time intervals remains a challenge. Over the last several years our approach to stroke patients has considered physiologic imaging parameters rather than the strict conventional time criteria. We found that patients treated late (>8 h) had no difference in outcomes than those treated early (≤8 h) if they were selected for treatment based on our physiologic imaging approach.
Intravenous tPA remains the mainstay for treatment of acute ischemic stroke. While it has been shown to be safe and relatively effective, many limitations exist. Only 4% of acute stroke patients receive intravenous tPA, mostly related to patients presenting beyond the 3 h time window.24 Among those patients presenting within the 3 h time window, nearly half are ineligible to receive intravenous tPA due to exclusionary criteria.24 Furthermore, intravenous tPA does not perform well in recanalizing and sustaining recanalization in large vessel occlusions.25 Nonetheless, in our practices all patients eligible for intravenous tPA still receive the drug prior to intervention.
Recanalization is critical in the acute treatment of ischemic stroke and it may be able to be used as a surrogate for good functional outcome and reduced mortality. A meta-analysis in 2007 demonstrated a nearly 4.5 increased odds of good functional outcome and 75% reduction in mortality in patients who were recanalized.26 The PROACT II trial first documented that stroke patients with successful recanalization, 66% of the trial's patients vs 18% no recanalization (p=0.001), had better clinical outcomes defined as mRS ≤2, 40% of those recanalized vs 25% of those achieving no recanalization (p=0.04).7 This was further supported by subgroup analysis from the multi-Merci trial where 49% of recanalized patients achieved mRS ≤ 2 compared with 10% of non-recanalized patients (p<0.001). This study also showed a significant increase in 90-day mortality in patients that were not recanalized compared to those that were able to be recanalized 52% vs 25% (p<0.001).23 Similarly, the Penumbra POST trial found that 45% of patients who were recanalized achieved mRS ≤ 2 compared with 13% of patients who were not successfully recanalized. We demonstrated similar rates of good functional outcome regardless of whether patients were treated endovascularly before or after 8 h from symptom onset. Our study found that 42.8% and 41.9% of patients treated before and after 8 h, respectively, achieved a 90-day mRS ≤ 2 (p=1.0). Also, when recanalization is considered, 71.7% patients treated ≤8 h from symptom onset achieved recanalization TICI 2b or 3 compared with 81.1% of patients treated >8 h from symptom onset (p=0.151).
However, in certain subsets of patients recanalization is not associated with improved functional outcomes and tissue perfusion may be a better marker both in selection of patients for treatment and in follow-up after treatment.27–29As such, physiologic imaging such as CTP is needed to better select and follow-up patients who derive more benefit from endovascular therapies. The use of CTP has been advocated over the last decade as a physiologic tool and, when used in conjunction with non-contrast CT, can identify the area of infarction through CBV maps and further define the penumbral region by integrating MTT and CBF data.30 The use of this physiologic approach to select patients within a specified time window is currently being studied in several randomized trials.31–33 This approach is also being applied to everyday practice to help select patients who present outside conventional time windows.34 We have similarly used this technique over the last several years to triage all stroke patients for potential IA therapy. We found that using CTP for selecting stroke patients allowed us to treat many patients who otherwise would not be considered candidates for treatment. This is best understood by comparing the two groups based on median time to presentation of less than or more than 8 h. When imaging criteria are used, 42.8% of patients treated ≤8 h from symptom onset achieved mRS ≤ 2 compared with 41.9% of patients treated >8 h from symptom onset (p=1.0). If we broaden our definition of good outcome to mRS ≤ 3, which still portends a functional lifestyle, in acute ischemic stroke, the same trend continues with 54.9% and 55.4% of patients treated ≤8 h and >8 h, respectively, achieving 90-day mRS ≤ 3 (p=1.0).27 ,29 ,35 ,36
Similarly, our overall mortality rate was 24.9% in patients treated ≤8 h and 20.3% in those treated >8 h from symptom onset, which is similar to previous stroke trials (HAMLET, DESTINY, PROACT, NINDS).1 ,7 ,35 ,36Additionally, our median admission NIHSS score was 17, demonstrating that our patient selection was not heavily weighted towards those with lower severity stroke. The causes for this favorable result in this instance are probably related to patient selection, where we followed prior European Cooperative Acute Stroke Study recommendations and excluded patients with large infarctions (more than one-third MCA territory) and selected those with relatively large penumbral volume as evidenced by CTP. Performing thrombectomy primarily with a device and then relative judicious use of intra-arterial tPA also likely contributes to low ICH rates. Close management in a dedicated neurocritical care unit may further contribute to improved outcomes.
Limitations of this study include the retrospective nature of data collection in a prospectively maintained patient database. Some patients had follow-up done through telephone interview and beyond 90 days, which could allow a bias to better outcome. The fact that we did not track the outcomes of the patients that we excluded based on penumbral imaging also does not allow us to compare outcome with this group. Additionally, the use of perfusion imaging for patient triage could potentially bias the early group, as some patients may have been excluded who would otherwise be included in comparative studies based on time parameters. The lack of a quantifiable methodology of CTP data for patient selection and the qualitative application of this approach by multiple physicians may weaken the applicability of this approach.
The practical application of physiologic imaging to select patients for acute stroke interventions irrespective of time was safe and effective in our multicenter experience, with patient outcomes similar to those seen in other acute stroke trials using time-based criteria. The strongest evidence is the similar rates of good clinical outcomes (mRS ≤ 2 and ≤ 3) in patients treated ≤8 h and those treated >8 h from symptom onset. The use of physiologic imaging in selecting patients for endovascular therapy is more patient-specific and may increase the number of eligible patients who could derive greater benefit from interventional therapies than time-guided criteria previously allowed.
References
Footnotes
-
Contributors Each author listed should receive authorship credit based on the material contribution to the article, their revision of the article and their final approval of the article for submission to this journal.
-
Funding None.
-
Competing interests None.
-
Ethics approval Ethical approval was obtained from the Institutional Review Boards at the Medical University of South Carolina, the University of Florida and the Swedish Medical Center.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.