Article Text
Abstract
Purpose To retrospectively evaluate the safety and efficacy of the endovascular treatment of wide-necked intracranial aneurysms assisted by a novel intra-/extra-aneurysm stent-like implant (pCONus).
Methods Initial and follow-up angiographic and clinical results are presented of 25 patients with 25 unruptured and ruptured wide-necked intracranial aneurysms treated by reconstruction of the aneurysm neck using the pCONus implant followed by coil occlusion of the fundus.
Results Successful intra-/extra-aneurysm deployment of the pCONus with coil occlusion of the fundus was achieved in all but one case. Procedure-related ischemic complications were observed in three cases with permanent deterioration in one. Acceptable aneurysm occlusion was achieved in all cases. Follow-up angiography revealed sufficient occlusion in 81.0% of the aneurysms. Intimal hyperplasia in the stented segment of the parent artery or device migration has not been observed to date.
Conclusions The pCONus device offers a promising treatment option for complex wide-necked bifurcation intracranial aneurysms. Acute or delayed dislocations of coils into the parent artery are successfully avoided.
- Aneurysm
- Coil
- Device
- Intervention
- Stent
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Endovascular treatment has developed into a safe and effective treatment option for both ruptured and unruptured intracranial aneurysms.1 Complex shaped wide-necked or fusiform aneurysms require modified endovascular techniques in order to maintain the patency of the parent artery. Several endovascular strategies have been established over the last few years, mainly consisting of innovative devices targeting the reconstruction of the aneurysm neck, the parent artery, or both. Stent-assisted coiling, flow diversion, the remodeling technique, or Y-stenting in bifurcation aneurysms are typically used.2–5 However, wide-necked aneurysms located at bifurcations with incorporation of parent arteries remain challenging even with the abovementioned technical modifications. The waffle-cone technique, consisting of implantation of a self-expanding stent into the afferent artery with its distal end in the aneurysm to provide stabilization before coil occlusion, was reported in 2007.6 ,7 An advancement of the waffle-cone technique resulted in the pCONus device, an electrolytically detachable stent with a distal crown and four petals deployed in the aneurysm with its base at the level of the neck, providing a more effective border to the parent artery than conventional stents.
In this paper we describe our initial experience with the pCONus device in the treatment of 25 wide-necked bifurcation aneurysms.
Materials and methods
pCONus device
The pCONus Bifurcation Aneurysm Implant (phenox GmbH, Bochum, Germany) is a stent-like intraluminal device for the treatment of wide-necked intracranial aneurysms (figure 1). The design allows for stable coil placement even in complex aneurysms by creating a mechanical barrier at the level of the aneurysm neck. The device is a laser cut nitinol structure consisting of three functional sections (figure 1). The most distal segment is the crown, which is composed of four opposing petals (loops) and serves as the weight-bearing surface for the coil mass. The second segment is the shaft, which anchors the stent to the afferent vessel. The device is available in six distal petal diameters of 5, 6, 8, 10, 12, and 15 mm. There are two versions, one with and one without a net of nylon fibers that prevents the coil mass from collapsing into the parent artery. The crown is deployed and positioned inside the aneurysm with the aid of four radiopaque markers mounted at the base of each petal. The shaft is a stent-like laser cut nitinol structure with low thrombogenicity due to a metal coverage area of <5%. The diameter of the shaft is 4 mm and it is available in 25 and 20 mm lengths. The third segment of the device is the cobalt chromium detachment zone connected to the insertion wire. A fifth radiopaque element visualizes the proximal end of the device under fluoroscopy. The separation of the implant from the insertion wire is accomplished with an electrolytic coil detachment system.
The pCONus device showing its three functional sections: crown, shaft, and detachment zone.
The pCONus can be deployed via a 0.021 or a 0.027 inch inner diameter microcatheter.
Patient population, inclusion and exclusion criteria
All medical records and radiographic studies of patients treated with the pCONus between February 2013 and September 2015 were analyzed and the results were entered into a database (Microsoft/Excel).
Patients signed an informed consent form at least 24 h prior to the procedure, apart from cases of acute subarachnoid hemorrhage from the target aneurysm. Inclusion criteria for endovascular treatment with the pCONus were wide-necked aneurysms located at vessel bifurcations incorporating the efferent parent arteries into the aneurysm base. All cases were discussed in a multidisciplinary vascular team with neuroradiologists, neurosurgeons, and neurologists.
The absolute exclusion criterion for endovascular treatment with the pCONus was intolerance or resistance to clopidogrel, ticagrelor, or acetylsalicylic acid (ASA).
There were 25 patients (18 women, 7 men) of mean age 61 years (range 44–77) with 25 intracranial aneurysms. The clinical pre- and post-procedural status of each patient was determined by the modified Rankin Scale (mRS).
Endovascular procedure
The procedures analyzed in this study were carried out under general anesthesia by two experienced interventional neuroradiologists.
The parent artery was catheterized with an 8F/6F telescoping guiding catheter combination or with a 6F catheter alone. An angulation without superimposition of the surrounding arteries was identified by three-dimensional rotational angiography. The diameter of the aneurysm neck was measured slightly above the transition zone to the parent artery and was used as the reference size for device selection. A 0.021 or 0.027 inch microcatheter was positioned in the center of the aneurysm assisted by a 0.014 or 0.016 inch microguidewire. Once the microcatheter was placed inside the aneurysm, the pCONus device was inserted and the crown was deployed by continuous withdrawal of the microcatheter without unsheathing of the shaft. The complete intra-aneurysm expansion of the petals and the centered orientation of the pCONus within the aneurysm was visualized under continuous fluoroscopy. The device was carefully pulled back to the aneurysm neck to create a stable border to the parent artery followed by further withdrawal of the microcatheter in order to deploy the shaft. At this point the device remained attached to the insertion wire.
A second microcatheter (Excelsior SL10; Stryker Neurovascular, Fremont, California, USA) was then placed within the aneurysm by navigation through the shaft of the pCONus followed by coil occlusion of the aneurysm. The border with the parent artery created by the pCONus was strictly respected. Coil loops that prolapsed below the crown were repositioned. After confirmation of satisfactory aneurysm occlusion, the microcatheter was withdrawn and the pCONus was electrolytically detached.
Antiplatelet and anticoagulation scheme
The regime of dual antithrombotic medication is similar to that for flow-diverters or intracranial stents in atherosclerotic disease, although the shaft of the pCONus device has a higher porosity than conventional stents and should be less thrombogenic.
A loading dose of 300 mg clopidogrel and 500 mg ASA was given at least 3 days prior to the procedure followed by a daily dose of 75 mg clopidogrel and 100 mg ASA in elective cases. Resistance to platelet anti-aggregation was tested using the impedance aggregometry method (Multiplate, Roche PVT GmbH, Waiblingen, Germany).8 ,9 The dual platelet anti-aggregation therapy was maintained for 3 months followed by monotherapy with 100 mg ASA daily. All procedures were carried out under systemic heparinization. In cases of acutely ruptured aneurysms, epifibatide was administered intravenously with a bolus followed by continuous intravenous application for at least 12 h bridged with an ASA and clopidogrel loading dose until sufficient dual antiplatelet platelet inhibition was achieved.
Follow-up schedule
Patients were scheduled for clinical and angiographic follow-up examinations 3 and 9 months after treatment. Clinical assessment was classified by the mRS by a neurologist or the interventionist. Angiographic results were graded according to the Raymond scale.10
Results
Aneurysm characteristics
The mean fundus diameter of the aneurysms was 9 mm (range 5–16 mm) and the mean neck diameter was 6 mm (range 4–13 mm). Sixteen aneurysms (64.0%) were located in the anterior circulation and nine (36.0%) were located in the posterior circulation (table 1).
Seven aneurysms (28%) were acutely ruptured. Eighteen patients (72.0%) were asymptomatic prior to the procedure, four (16.0%) had an mRS score of 1 or 2, and three patients (12.0%) had an mRS score of ≥3, each caused by a previously ruptured target aneurysm.
Eighteen of the aneurysms were initially treated with the pCONus device, four of the aneurysms in this series were remnants of previously coiled aneurysms, two were previously treated with an intra-aneurysm flow diverter (WEB device), and one had been partially clipped.
Procedural technical aspects and difficulties
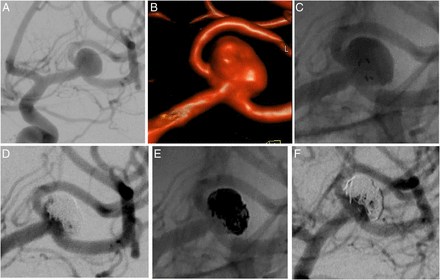
Deployment and detachment of the pCONus device with proper reconstruction of the parent arteries was successful in 24 cases (96.0%) (figure 2A–F).
(A) Incidental finding of a wide-necked middle cerebral artery aneurysm on the left side in a 53-year-old woman (right anterior oblique and caudal view). (B) Three-dimensional reconstruction of the same aneurysm. (C) Positioning of a 4-25-5 mm pCONus device via a 21 inch microcatheter within the aneurysm and along the afferent artery. Correct expansion of the four petals by the arrangement of the radiopaque markers (right anterior oblique and caudal view). (D) Coil occlusion of the aneurysm with a microcatheter placed inside the aneurysm after expansion and before detachment of the pCONus with a small remnant at the caudal circumference (right anterior oblique and caudal view). (E) Complete coil occlusion of the aneurysm after repositioning of the microcatheter followed by detachment of the pCONus device (right anterior oblique and caudal view). (F) Three-month follow-up angiography showing a small neck remnant (right anterior oblique and caudal view).
The final detachment failed in one case of a ruptured basilar artery aneurysm after complete coil occlusion so the pCONus was removed without resheathing into the microcatheter to avoid dislocation of the coil mass. Nevertheless, a single coil loop prolapsed into the origin of the posterior cerebral artery and a mild dissection of the vertebral artery in the V4 segment occurred. The reason for the detachment failure could not be determined (figure 3A–E).
(A) Ruptured basilar tip aneurysm in a 63-year-old man (anteroposterior view with cranial angulation). (B, C) Placement of a 0.021 inch microcatheter inside the aneurysm followed by positioning of a 4-25-10 mm pCONus with its crown inside the aneurysmal orifice. Catheterization through the shaft of the pCONus inside the aneurysm. (D) Displacement of a single coil loop into the origin of the posterior cerebral artery after failed detachment of the pCONus device followed by retrieval without resheathing into the microcatheter. (E)Dissection of the vertebral artery caused by retrieval of the pCONus device without hemodynamic consequences.
Peri- and post-procedural complications
The acute peri- and post-procedural phase was without procedure-related clinically relevant complications in 22 cases (88.0%). Two patients had minor ischemic lesions with transient neurologic deficits. One patient with an incidental basilar artery aneurysm deteriorated clinically due to a post-procedure infarction in the posterior cerebral artery. Two patients died due to consequences arising from a subarachnoid hemorrhage at the time of admission (table 2).
Delayed complications
Neither aneurysm rupture nor occlusion of the parent artery or delayed thromboembolic events were observed during the follow-up period.
Angiographic results
Complete initial occlusion of the aneurysmal sac was achieved in 10 cases (40.0%). Twelve patients (48.0%) had a neck remnant and in three (12.0%) a small fundus perfusion remained. The first follow-up angiography was carried out after a mean of 3.5 months in 21 patients (84.0%). More than one follow-up examination was performed in 13 patients with a mean interval to the latest examination of 15.4 months. The mean follow-up period of the entire series is 10.2 months to date (table 3).
Illustrative cases
Illustrative cases are shown in figures 2A–F and 3A–E.
Discussion
Coiling or clipping are accepted treatment options for endovascular treatment of both ruptured and unruptured aneurysms.11 Wide-necked aneurysms, especially those located at bifurcations, remain difficult to treat since dense coil packing is usually impossible due to the missing abutment represented by a defined neck. The width of the aneurysm neck becomes significant in relation to the aneurysm dome. Brinjikji et al12 analyzed the dome-to-neck ratio of 185 endovascularly treated intracranial aneurysms and identified a dome-to-neck ratio of <1.2 as an independent predictor of the need for adjunctive endovascular techniques (stent or balloon).
There are several endovascular techniques that address the issue of wide-necked aneurysms.13–19 Pierot et al performed a literature review of the safety and efficacy of the balloon remodeling technique and found a similarity in the safety profile compared with standard coiling. The immediate and follow-up results in the remodeling group were even superior to those in the standard coiling group.15 However, the treatment of broad-based bifurcation aneurysms remains technically challenging, especially in cases of complex anatomy. In a series of 19 coil embolizations of wide-necked aneurysms using double stents in a Y configuration presented by Spiotta et al,20 the incidence of complications at initial treatment was 31.6% and delayed thromboembolic complications occurred in 10.5%. These data indicate the technical complexity of the treatment of wide-necked aneurysms. The efferent arteries originating from the neck area of the aneurysm are difficult to visualize without superimposition of the aneurysm, and selective catheterization of the efferent artery for stent placement is often difficult or impossible due to an unfavorable angle and the wide neck. This problem was approached by the ‘wire anchor loop traction maneuver’ described by Effendi et al.21 The technique consists of catheterization of the efferent artery through the aneurysm with a loop that is then straightened by repeated traction of the microcatheter. The distal part of the microcatheter can be stabilized by a balloon or stent acting as an anchor (‘balloon or stent anchor technique’). However, these maneuvers might increase the risk of aneurysm rupture since the delivery catheter needs to be pushed against the aneurysm wall.
A technique that characterizes the principle of the pCONus-assisted technique was presented as the waffle-cone technique.6 ,22 The distal end of a self-expanding stent is placed inside the aneurysm orifice with its middle to proximal section inside the afferent artery. The cone-shaped end of the stent allows for preservation of the afferent and efferent parent artery during and after coil occlusion of the aneurysm via a microcatheter placed along the stented afferent artery.
Our results demonstrate a comparatively safe and efficient treatment option for wide-necked bifurcation aneurysms by the use of the pCONus device without the need to catheterize the efferent arteries. We observed one clinically relevant complication (patient 1) and two cases of transient ischemic symptoms that were most probably not directly related to the device (patients 20 and 25). Aguilar Pérez et al23 presented the first series of 28 intracranial aneurysms treated with the pCONus device and found three patients with transient ischemic symptoms and no case of permanent neurologic deficit. Their initial angiographic results are comparable to our findings, with 28.6% complete occlusion compared with 40.0% in our series. We did not observe angiographic improvement in the follow-up period in cases with remaining remnants; this differs from the findings of Aguilar Pérez et al who found a spontaneous improvement of at least one point on the Raymond grading scale in nine cases. These findings might explain the poorer rate of complete occlusion in the follow-up period in our series (33.0% vs 59%). We observed four cases of slightly increased reperfusion; all were cases of comparatively large aneurysms with incomplete initial occlusion.
Four of the aneurysms in our series were included in a multicenter analysis of 40 middle cerebral artery aneurysms treated with the pCONus device.24 The initial angiographic results were in line with our results and those of Aguilar Pérez et al, with a complete occlusion rate of 25% and a neck remnant in 47.5%. Again the rate of complete occlusion improved to 48.5% in the follow-up period in this series. The rate of adequate occlusion in this series (total occlusion and neck remnant) was comparable to our results (78.8% vs 81.0%).
In a recently published study by Lubicz et al25 of 19 unruptured aneurysms treated with the pCONus device with promising initial complete occlusion rates (68.4% complete occlusion), three recanalizations occurred during a mean follow-up period of 9.5 months. These results are similar to those of our series with four reperfusions. Retreatment presented no difficulty as the pCONus device could be easily accessed with the microcatheter and the parent artery was protected by the device, as in the initial procedure.
The PulseRider device is a self-expanding nitinol implant that can be repositioned and torqued to fit the relevant anatomy of wide-necked bifurcation aneurysms. It has 5–7% metal to artery coverage since the majority of the surface area coverage is focused at the neck of the aneurysm, a factor that might allow for a reduction in the concomitant dual antiplatelet therapy.26
Piotin et al27 presented eight cases of wide-necked bifurcation aneurysms treated with the Barrel device, a stent with a barrel-shaped middle section to reconstruct the aneurysm neck by stenting the more involved parent artery. This effect can also be accomplished with a conventional self-expanding stent described by Darflinger et al28 as the ‘barrel technique’. These techniques may provide sufficient coverage of the aneurysm neck without the need to access the aneurysm with a 0.021 or 0.027 inch microcatheter that might potentially increase the risk of rupture. Nevertheless, no cases of rupture or dissection were reported in the pCONus series or in the results of the WEBCAST study in which 51 aneurysms were treated with the WEB device, an intra-aneurysmal implant designed for the treatment of wide-necked aneurysms placed via a 0.027 inch microcatheter.29
In summary, our results are mostly comparable to the published data even though there are minor differences in the angiographic outcome. We think the results confirm the justified value of the pCONus device in the growing field of neck bridging devices.
Limitations of the study
Our study has several limitations, including the single-center retrospective design and the absence of a core laboratory for an independent analysis of the angiographic and clinical data. The pending long-term follow-up results prevent a final assessment of the presented treatment.
Conclusion
The pCONus device offers a promising treatment option for complex intracranial wide-necked bifurcation aneurysms. Acute or delayed dislocations of coils into the parent artery are successfully avoided. Long-term follow-up data are needed to confirm the permanent effect of the technique.⇓⇓⇓
Demographic data and aneurysm characteristics
Summary of peri- and post-procedural complications
Angiographic follow-up results
Acknowledgments
The authors are most grateful to James Lago for language revision of the manuscript.
References
Footnotes
Contributors SF: conception and design of the work; acquisition and analysis of the data, interpretation of data, manuscript preparation. AW, AT, CB, AK: drafting the work and critical revision. WW: manuscript preparation and critical revision of the manuscript.
Competing interests SF: support for travel to meetings: phenox; travel expenses for the meeting of the German Society of Neurosurgery 2015. WW: consultancy: Proctor for the pCONus and p64 Flow Modulation Device; payment for lectures (including service on speakers’ bureaus): phenox (products: pCONus and flow-diverter p64 Flow Modulation Device).
Ethics approval Ethics approval was obtained from the local ethical review committee.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All data from this study are contained in this paper.