Article Text
Abstract
Background Recent studies have validated the use of endovascular thrombectomy in large vessel ischemic stroke provided patients are selected appropriately. However, to our knowledge, there have been no previously reported cases of endovascular thrombectomy in patients with aortic dissection. We report three such cases, two with chronic aortic dissections (including one with a history of Marfan syndrome) and another with an acute type B dissection.
Methods Case studies and review of relevant literature.
Results Three patients with a history of aortic dissection presented with acute onset right middle cerebral artery syndromes, two of whom had chronic aortic dissections that were status-post graft repair, while a third had an acute type B aortic dissection that had been managed with a femoral-to-femoral bypass. None of the three were candidates for intravenous tissue plasminogen activator. All three were found to have proximal right M1 occlusions on non-invasive imaging and were taken for endovascular thrombectomy via transfemoral, transradial, and transbrachial approaches, respectively. All three had successful recanalization (with Thrombolysis In Cerebral Infarction (TICI) 2b, TICI 3, and TICI 2b flow, respectively) along with clinical improvement, and none had procedure-related complications.
Conclusions These three cases suggest that endovascular thrombectomy is feasible and can be done safely and efficaciously in patients with aortic dissections and those with Marfan syndrome, although the risks and benefits should be considered as part of any decision-making process. Given that endovascular therapy for acute stroke is now in many situations part of standard care, further studies will be necessary to delineate more precise inclusion and exclusion criteria.
- Dissection
- Intervention
- Stroke
- Thrombectomy
Statistics from Altmetric.com
Background
Recent studies have validated the use of endovascular thrombectomy in large vessel ischemic stroke provided patients are selected appropriately, with an efficacy significantly greater than that of thrombolysis alone.1–5 However, it is unclear whether its use can be extended to occlusions caused by arterial dissections, the most challenging of which may be those of the thoracic aorta. Patients with aortic dissection may have strokes from multiple mechanisms, including occlusion from direct extension into the carotid arteries, border zone infarcts from hemodynamic instability and decreased cerebral perfusion pressure, and thromboembolism.6
While one of the exclusion criteria for the EXTEND-IA study was angiographic evidence of carotid dissection, it was left to the discretion of the treating physician in MR CLEAN, and was not mentioned at all in the protocol for ESCAPE or SWIFT-PRIME; REVASCAT excluded carotid dissections only if they prevented access to the target vessel and could not be simultaneously treated. Further, only SWIFT-PRIME explicitly excluded any patient with a suspicion of aortic dissection.
Although a retrospective study and review of endovascular thrombectomy in carotid artery dissection appeared to show a trend toward benefit over intravenous tissue plasminogen activator (IV tPA) alone,7 to our knowledge there have been no previously reported cases of endovascular thrombectomy in patients with aortic dissection. Open intraoperative thrombectomy of carotid occlusions in the setting of acute aortic dissection has been described (via median sternotomy, cardiopulmonary bypass, and hypothermic circulatory arrest), albeit with mixed success.8
Here we report three cases of successful recanalization with endovascular thrombectomy in patients with aortic dissection, without procedure-related complications.
Case 1
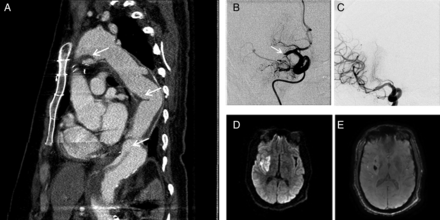
A patient with a history of Marfan syndrome, chronic aortic dissection status-post ascending aortic aneurysm graft repair, and mechanical aortic valve on warfarin was found in a car after a low impact motor vehicle accident. Upon arrival to a local hospital the patient was noted to be obtunded, flaccid on the left side with a right gaze deviation and garbled speech; NIH Stroke Scale (NIHSS) score was 25. Head CT showed an ill-defined hypodensity in the right basal ganglia with loss of the insular ribbon but an otherwise favorable ASPECTS score, while CT angiography of the head showed a proximal right middle cerebral artery (MCA) occlusion. Other scans also redemonstrated the patient's known aortic dissection and repair (figure 1A). The patient's INR was noted to be subtherapeutic; however, because the time of symptom onset was unknown, tPA was not given.
(A) CT angiogram of the chest, sagittal view, demonstrates aortic valve and ascending aortic graft repair as a single conduit, with reimplantation of the coronary arteries; a residual dissection flap along the lesser curvature of the aortic arch with aneurysmal dilation and a false lumen with mural thrombus (arrow); and a descending thoracic aortic graft repair, beginning at the isthmus and extending to the lower descending thoracic aorta, with mural thrombus present within the graft, and an area of ulcerated thrombus in the proximal one-third of the graft (arrow). There is a residual communicating aortic dissection beginning just distal to the termination of the descending thoracic graft where there is a large re-entry tear with extension to the proximal infrarenal aorta (arrow). (B, C) Digital subtraction angiography, anteroposterior view. Right internal carotid (B) arteriogram demonstrates proximal right middle cerebral artery (MCA) occlusion (arrow). Subsequent mechanical thrombectomy was performed which yielded Thrombolysis In Cerebral Infarction 2b flow (C). (D, E) MRI brain, axial views. Diffusion-weighted imaging (D) shows acute infarct in the core of the right MCA territory. Susceptibility-weighted imaging (E) shows petechial hemorrhage in the basal ganglia.
The patient was transferred to a tertiary academic medical center for further management, where his examination remained unchanged on arrival. Given the small infarct core on head CT, proximal occlusion, and persistent severe neurological deficit, a multidisciplinary team including the patient's cardiothoracic surgeon determined that the possible benefits of endovascular intervention outweighed the risks, and the patient was taken urgently for mechanical thrombectomy. Using a transfemoral approach, a 6 Fr sheath was advanced into the right common femoral artery then substituted for a 6 Fr Neuron MAX sheath (Penumbra, Alameda, California, USA), which was advanced to the level of the aortic arch before further advances into the common carotid artery were made with a 5 Fr VERT catheter (Cook Medical, Bloomington, Indiana, USA). Once the right MCA occlusion was reached, the neuron MAX was placed first in the distal right common carotid artery, then the proximal internal carotid artery, and a 5 MAX ACE aspiration catheter/velocity microcatheter combination was advanced distally across the occlusion. After two attempts at deploying a Solitaire stentriever (Covidien/ev3, Irvine, California, USA) along with manual aspiration, the vessel was successfully recanalized with TICI 2b flow (figure 1B, C).
After the procedure the patient's NIHSS improved to 11, with some movement noted in the left arm and leg, complete resolution of the sensory loss and gaze deviation, and partial improvement of the hemineglect. MRI subsequently showed a relatively moderate infarct in the basal ganglia and portions of the inferior MCA territory on the right, as well as mild deep petechial hemorrhage (figure 1D, E).
After 5 days anticoagulation was restarted as the risk of further stroke from the patient's mechanical aortic valve was deemed to be higher than the risk of further hemorrhagic conversion. A heparin infusion was started, then transitioned to warfarin; 2 days later there was a worsening of the patient's examination with re-emergence of a gaze deviation, visual field cut, and hemisensory loss. This prompted a repeat head CT which showed some mild expansion of the hemorrhagic conversion and evolution of the inferior division right MCA stroke; however, serial scans and examinations remained stable over the following 2 days and the patient was discharged to an inpatient rehabilitation facility. At 4-month follow-up the patient's NIHSS had improved to 5 with residual left hemiparesis and hemisensory deficit, and the modified Rankin Scale (mRS) score had improved to 2.
Case 2
A patient with a history of a type A aortic dissection status-post endograft repair had a witnessed collapse and was found to have severe weakness of the left side. Upon arrival to a tertiary academic medical center the patient's examination was noted to have improved significantly from the initial report, with a documented initial NIHSS of 2. As a result, the patient was deemed not to be a candidate for IV tPA. However, while still in the emergency department there were fluctuations in the patient's symptoms, with the NIHSS initially going up to 9, improving to 5 after administration of an IV fluid bolus, and then increasing to 10 again shortly thereafter, with a right gaze preference, significant weakness of the left side, decreased sensation, and hemineglect. Head CT had shown no evidence of hemorrhage, but by this point the patient was out of the tPA window; meanwhile, the ASPECTS score was 9 and CT angiography showed a proximal right MCA occlusion without any evidence of new changes to the patient's repaired aortic dissection.
Further attempts were made to augment the patient's cerebral perfusion with IV fluids but without any further improvements in clinical status. Neurologic worsening was attributed to failure of collateral vessels and, given the expected poor outcome without recanalization, the patient was taken emergently for endovascular thrombectomy. A transradial approach was taken, after which several attempts to negotiate the right common carotid artery with SIM 2 and VTK catheters (Cook Medical) failed due to tortuosity in the innominate artery. Instead, a Shetty catheter (Cook Medical) was used in the innominate artery to advance an 18 L microcatheter over a Synchro microwire (Stryker Neurovascular, Fremont, California, USA) into the right internal carotid artery. A triaxial system consisting of a 5 MAX ACE over the 18 L microcatheter and Synchro microwire was used to cross the site of occlusion; a Solitaire device was then deployed, followed by manual aspiration at the site of occlusion with the 5 MAX ACE tracked over the Solitaire device. Complete recanalization in the proximal MCA and both superior and inferior branches was achieved, consistent with TICI 3 flow (figure 2). After the procedure the patient's NIHSS improved to 4 without any post-procedural complications. The mRS score was 0 at 90 days.
Catheter-based angiogram, anteroposterior view. (A) An aortic graft repair is initially visible (arrow), along with sternotomy wires. Right internal carotid arteriogram (B) demonstrates proximal right middle cerebral artery occlusion (arrow). Subsequent mechanical thrombectomy was performed which yielded Thrombolysis In Cerebral Infarction 3 flow (C).
Case 3
A patient with a history of hypertension and tobacco use was admitted to a tertiary academic medical center for acute leg ischemia in the setting of a type B aortic dissection (extending from the left subclavian artery down to the iliac arteries), which was treated with a left-to-right femoral-to-femoral crossover bypass. Overnight on the first postoperative day the patient developed an acute onset of altered mental status. The primary surgical service obtained a non-contrast head CT overnight which was unrevealing; formal neurologic evaluation was not requested until the following morning, at which point the patient was also noted to have significant left-sided weakness in addition to persistent confusion, with NIHSS 10. A brain MRI was obtained later that morning and showed an evolving infarct in the internal border zone of the right hemisphere, while a brain MR angiogram revealed a right M1 occlusion (figure 3A, B).
MRI and MR angiogram of the brain (A, B), digital subtraction angiography, anteroposterior view (C, D). Axial diffusion-weighted imaging (A) shows internal border zone infarcts in the right centrum semiovale, while MR angiography (B) shows a right M1 cut-off (arrow). Right internal carotid arteriogram (C) demonstrates proximal right middle cerebral artery occlusion (arrow). Subsequent mechanical thrombectomy was performed which yielded Thrombolysis In Cerebral Infarction 2b flow (D).
Given the relatively small infarct burden and the likelihood that the patient would progress to develop further infarcts in the right MCA territory without intervention, the patient was taken for mechanical thrombectomy after discussion with the primary surgical service. A right transbrachial approach was used with a 6 Fr sheath, with subsequent uncomplicated access to the right M1 occlusion using a triaxial system consisting of a 5 MAX catheter over an 18 L microcatheter and 0.014 inch Synchro microwire. After three attempts were made at manual aspiration, successful TICI 2b recanalization was achieved (figure 3C, D). Repeat brain MRI did not show any new areas of infarct and over the next several days the patient's symptoms slowly improved. At outpatient follow-up 1 month later the NIHSS score was down to 3 and the mRS score was 1 at 90 days.
Discussion
Our case series suggests that endovascular thrombectomy is feasible and can be done safely and efficaciously in patients with aortic dissections, including those with Marfan syndrome as in the first case. As with any other medical situation, however, the risks and benefits should be considered as part of any decision-making process, and the risk of such a procedure in the setting of pre-existing dissection is likely to be higher than that in the general population.
Extrapolating from the cardiac literature, catheterization in patients with aortic dissection may be technically difficult, time consuming, and potentially risky, as standard techniques may not allow access to the true lumen of the aorta. Further, there is also the risk that advancing the catheter or guidewire may cause extension of the dissection, displacement of thrombotic material, or outright perforation of the aorta.9 Previous authors have suggested that such technical issues can be circumvented by first evaluating the patient's pulses, reviewing other diagnostic imaging, and using larger sheaths and guidewires to allow better visualization and torque control.10 ,11 The access point is also a major consideration and must be decided on a case-by-case basis—for example, three different approaches were used in these three cases (transfemoral, transradial, and transbrachial, respectively). The major factors influencing choice of access site include the location and acuity of the dissection as well as the location of the target vessel. For example, an acute dissection may prohibit a transfemoral approach, particularly if access to the target vessel is limited by a false lumen, whereas a more chronic dissection would likely be healed, making a transfemoral approach more technically feasible. Meanwhile, if the target vessel is in the anterior circulation, a direct carotid approach may be favorable, although closure is a challenge with transcervical access in uncooperative patients and those exposed to thrombolytic agents.11 In such situations, and in target vessels in the posterior circulation, a transbrachial or transradial approach may be more appropriate.
Despite these possible risks, one review suggests the potential safety of catheterization in such situations, citing an unpublished report of 20 patients with aortic dissection who received coronary angiograms.12 The only complications in that series were a brachial artery occlusion requiring surgical repair and two patients whose coronary ostia were unable to be engaged. The key is that the situation must dictate that any potential benefit outweighs the risk. In each of our cases there was a high likelihood of poor outcome without recanalization in the setting of proximal occlusion and persistent severe neurologic deficits, while the risk was not considered prohibitive. Given that endovascular therapy for acute stroke is now in many situations part of standard care, further prospective studies will be necessary to delineate more precise inclusion and exclusion criteria, both for those patients who may not derive as much benefit from the procedure and those whose overall medical condition presents excessive risk.
References
Footnotes
Contributors MER, APJ, and MAJ conceived the study. MER and APJ initiated the study design. AES, MAJ, and AFD helped with implementation.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.