Article Text
Abstract
Background The Pipeline embolization device has been used successfully to treat intracranial aneurysms with difficult morphologies. However, the need for dual antiplatelet therapy has limited its use after subarachnoid hemorrhage.
Case report A 42-year-old woman with a ruptured dissecting aneurysm of her dominant vertebral artery (V4) was successfully treated by Pipeline embolization with preservation of flow through a covered posterior inferior cerebellar artery. This strategy preserved endovascular access for the treatment of severe posterior circulation vasospasm. She was a non-responder to thienopyridine agents and was thus maintained on aspirin and heparin, which was transitioned to warfarin following ventricular drain removal. The aneurysm remains angiographically obliterated at 6 months. Despite a moribund presentation and an extended hospitalization, she has made a remarkable neurological recovery.
Conclusions Pipeline embolization may be used to treat a ruptured dissecting aneurysm in selected cases where parent vessel preservation is paramount.
Statistics from Altmetric.com
Background
Endovascular flow diversion has emerged as an effective strategy for treating intracranial aneurysms with challenging morphologies.1 The Pipeline Embolization Device (PED, EV3, Irvine, California, USA) was recently approved for the treatment of wide-necked internal carotid artery (ICA) aneurysms and has also been successfully used to treat a wide variety of aneurysms.2
Dissections of the intracranial vertebral artery (VA) may cause subarachnoid hemorrhage (SAH), and endovascular treatment of these aneurysms often involves parent vessel occlusion. When contralateral blood flow is inadequate or dissection involves the origin of the posterior inferior cerebellar artery (PICA), endovascular reconstruction of the injured segment may obliterate the aneurysm while preserving parent vessel flow.3 Following acute SAH, however, the use of antiplatelet agents may increase hemorrhagic complications and complicate management of cerebrospinal fluid diversion.
We report a ruptured VA dissecting aneurysm that was treated successfully with Pipeline embolization.
Case report
A 42-year-old woman arrived comatose at our institution with diffuse SAH. CT angiography showed a dissecting aneurysm of the dominant left VA (figure 1A). Her hemodynamic status rapidly deteriorated causing cardiac arrest. Following resuscitation, an intra-aortic balloon pump was placed.
(A) CT angiographic three-dimensional reconstruction showing a dissecting aneurysm of the left vertebral artery (VA) (arrow) distal to the posterior inferior cerebellar artery (PICA) takeoff. The right VA is hypoplastic. (B) Lateral projection of a right VA injection confirms the hypoplastic nature of the right VA with minimal contribution to the basilar artery (arrow). The anterior spinal artery (ASA) is visible on this projection (double arrows). (C) Working angle projection of the left VA injection more clearly demonstrates the extent of VA injury and its relationship to the PICA origin (arrow). The ASA (double arrows) is also visualized on this injection. Used with permission from Barrow Neurological Institute.
She had a hypoplastic right VA (figure 1B), a fetal right posterior cerebral artery (PCA) and a small left posterior communicating artery, and occlusion of the left VA would risk catastrophic stroke. Given her cardiac dysfunction, surgery was not considered. After extensive discussion it was decided to proceed with endovascular flow diversion.
A left VA angiogram revealed a V4 segment dissection beginning immediately distal to the PICA (figure 1C). She was heparinized and the diagnostic catheter was exchanged for a 6F guide catheter (Neuron, Penumbra, Alameda, California, USA). A Marksman catheter (EV3) over a 0.014-Synchro-2 microwire (Boston Scientific, Natick, Massachusetts, USA) was advanced to the distal basilar artery. A 3 × 16 mm PED was deployed, landing just distal to the dissection (figure 2A,B). This device did not cover the proximal dissection, so a second (3.25 × 16 mm) device was deployed in an overlapping fashion (figure 2C). Control angiography revealed persistent aneurysmal filling and robust PICA flow (figure 2D). Following groin hemostasis, abciximab (0.125 mg/kg) was administered intravenously, followed 3 h later by 325 mg acetylsalicylic acid and 450 mg clopidogrel.
(A) Unsubtracted image of working angle projection after deployment of the first device (arrow). (B) Left vertebral artery (VA) injection after deployment of the first device. The aneurysm continued to fill and the first device did not cover the proximal aspect of the dissection, represented by the ‘notch’ (arrow) opposite the origin of the posterior inferior cerebellar artery (PICA). (C) Unsubtracted image after deployment of the second device (arrow). (D) Control VA angiogram after deployment of the second device which now covers the proximal aspect of the dissection as well as the PICA origin. Note the continued filling of the PICA (arrow). Used with permission from Barrow Neurological Institute.
Platelet inhibition assays (VerifyNow, Accumetrics, San Diego, California, USA) performed at regular intervals over the first 16 days revealed a lack of response to clopidogrel, prasugrel and ticlopidine, although she was responsive to aspirin. She was reheparinized on postoperative day (POD) 1 and transitioned to warfarin following ventricular drain removal. Angiography on POD 7 confirmed aneurysm exclusion and the intra-aortic balloon pump was removed. On POD 8, MRI revealed punctate infarcts in the superior vermis, bilateral cerebellar hemispheres and bilateral occipital lobes without brainstem infarction. On POD 9, repeat angiography revealed severe vasospasm, prompting balloon angioplasty of both ICAs, the left VA and basilar artery (figure 3A,B).
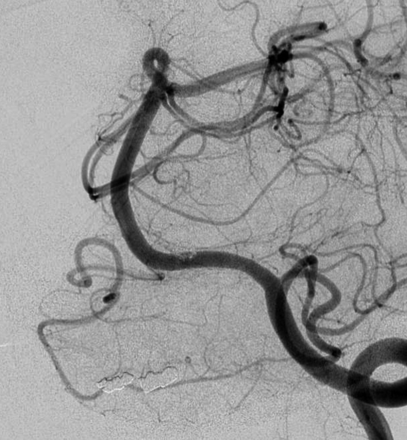
(A) Left vertebral artery (VA) injection on day 9 showing extensive vasospasm of the posterior circulation. Note the severe constriction of the VA at the proximal end of the device (arrow) and diffuse spasm of the basilar artery (double arrows). (B) Left VA injection after extensive balloon angioplasty showing a marked increase in vessel diameter. Used with permission from Barrow Neurological Institute.
She ultimately underwent tracheostomy, gastrostomy and delayed ventriculoperitoneal shunt placement and was discharged to rehabilitation on POD 30. At that time she followed commands with right hemiparesis. Warfarin was stopped at 3 months and aspirin was continued. At 6 months, repeat angiography revealed stable aneurysm occlusion (figure 4). She exhibited only mild short-term memory deficits.
Left vertebral artery (VA) injection with original working angle projection performed 6 months after initial treatment. The aneurysm remains stably occluded without significant device stenosis and with flow preserved through the left posterior inferior cerebellar artery. Used with permission from Barrow Neurological Institute.
Discussion
Dissecting VA aneurysms exhibit a high risk of recurrent hemorrhage.4 Treatment of these lesions usually involves microsurgical trapping, wrapping of the diseased segment or coil embolization. Occlusion may be accomplished proximally, both proximally and distally to the site of injury, or along the entire diseased segment. Such deconstructive approaches may necessitate a bypass when the dissection involves the PICA origin or when contralateral VA flow is limited.5
Reconstructive procedures using stents, with or without adjunctive coil embolization, have also been used to treat ruptured VA dissections. Stent placement requires antiplatelet agents which increase the risk of hemorrhage. Park et al 3 described 11 ruptured vertebrobasilar dissecting aneurysms treated by standalone placement of balloon-expandable coronary stents.3 Dual antiplatelet agents were administered immediately after the procedure and continued for 3–6 months. Heparinization was maintained for 24–48 h. One patient experienced fatal rebleeding.
Endovascular flow diversion using the PED represents a theoretical advance in the treatment of dissecting aneurysms. The decreased porosity and increased surface area of these devices facilitate aneurysm exclusion while preserving important side branches such as the PICA or anterior spinal artery. Furthermore, preserving flow through the VA maintains endovascular access to the posterior circulation for treatment of vasospasm. Several reports demonstrate the efficacy of PED placement for intracranial VA dissections.6 ,7 Reports of treating ruptured aneurysms with the PED are scarce,8–10 but de Barros Faria et al 7 reported four patients with ruptured VA dissections. Dual antiplatelet agents were maintained for 6 months and complete occlusion (3–6 months) was reported for three cases. Narata et al 10 described two patients with ruptured VA dissections, each treated by the delivery of three devices. In both cases the aneurysms were distant from the PICA. PED placement led to immediate aneurysm exclusion in one case and exclusion at 48 h in the other. By 3 months, no procedural morbidity or rebleeding was observed.
In our case, two PEDs were employed, the second of which ‘jailed’ the left PICA. Aneurysm exclusion was observed by POD 7. Although reports of post-treatment hemorrhage are rare, it is unclear how rapidly flow diversion obviates the risk of re-hemorrhage, particularly following antiplatelet therapy. Our case also highlights pitfalls in managing antiplatelet agents. After it was found that she did not respond to clopidogrel (and later prasugrel and ticlopidine), we elected to heparinize and transition to warfarin. We believe that attention to antiplatelet responsiveness is critical in minimizing thromboembolic complications associated with PED placement.
Despite a moribund presentation, our patient made a remarkable recovery. This case demonstrates the use of the PED to treat a ruptured dissecting aneurysm while preserving the parent artery. To our knowledge, it is the first reported case in which the PED spanned the PICA in a ruptured VA dissection. Given the unknown risk of re-hemorrhage after such treatment, patients and families must be advised regarding surgical and endovascular alternatives. Further optimization of antiplatelet agents following SAH may minimize the risks of thromboembolic and hemorrhagic complications.
References
Footnotes
-
Contributors All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
-
Competing interests None.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.