Article Text
Abstract
Background Challenging anatomy for carotid artery access can result in a delay to achieve successful recanalization in patients with acute ischemic stroke. Our objective was to study emergent direct percutaneous carotid artery puncture as an alternative access approach for acute endovascular stroke interventions.
Methods We reviewed cases of acute ischemic stroke in which direct carotid artery puncture was used for access. We also reviewed current literature relevant to this subject.
Results We describe the technical aspects, limits, and potential complications associated with direct carotid artery puncture for intracranial acute ischemic stroke interventions, and present cases to illustrate the utility of this access approach.
Conclusions Direct carotid artery puncture is a feasible alternative to transfemoral artery access in cases of stroke with difficult anatomy, including unfavorable arch type, carotid tortuosity, or an ostial lesion.
- Artery
- Technique
- Stroke
Statistics from Altmetric.com
Introduction
Shorter procedure times in patients with acute ischemic stroke undergoing endovascular intra-arterial intervention are strongly associated with good clinical outcomes.1 ,2 Difficult anatomy, such as unfavorable elongated aortic arch or severe tortuosity, or ostial stenosis of the proximal cervical vasculature can significantly delay or even preclude guide catheter access to the carotid artery and is associated with unfavorable clinical outcomes in patients with acute stroke.3
For aneurysm coil embolization, arteriovenous malformation embolization, or carotid artery stenting (CAS) procedures in which the standard percutaneous transfemoral route cannot be established because of unfavorable anatomy, alternative approaches such as direct transcervical or transbrachial access can be utilized.4–7 However, in most cases, these are performed under elective and preplanned conditions. The patient population requiring acute stroke intervention is older and more likely to have tenuous proximal vascular access.
Here we present our initial experience with obtaining emergent direct percutaneous carotid artery access for acute endovascular stroke interventions. We review technical aspects and present cases illustrating the indications, limitations, and potential complications associated with this alternative transcutaneous arterial approach.
Technique
Indications
Anatomical variants associated with technically challenging carotid access have been extensively described in the literature, mostly in conjunction with cases of CAS.8 ,9 Such variants include any of the following: bovine arch; type II and especially type III arch; tortuosity, severe angulation, or loops of the common carotid artery; and common carotid artery ostial stenosis. At our institution, emergent CT angiography of the head and neck is performed on every patient considered for endovascular stroke intervention. We proceed with carotid artery puncture for endovascular access when several of the above anatomical variants are found on the CT angiogram.
Technique description
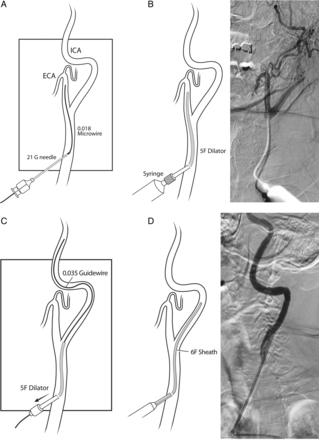
Direct carotid access can be obtained with the patient under general anesthesia or with conscious sedation. The main steps of this technique are illustrated in figure 1. Using ultrasound to visualize the carotid artery and its bifurcation, the intended target puncture site is located 3–4 cm above the clavicle. The area is prepared and draped, paying attention to avoid direct coverage of an awake patient's face with the drapes. The entry site is infiltrated with 5–8 mL of local anesthetic (2% lidocaine). Using a 21 gauge micropuncture needle, the common carotid artery is punctured at a 45° angle under ultrasound guidance to avoid entry into the overlying jugular vein. An angiographic run is performed through the micropuncture needle to evaluate the entry site and bifurcation anatomy, as well as create a road map. A floppy tip 0.018 inch soft tip microwire is advanced under direct fluoroscopic roadmap (figure 1A). We navigate the microwire into the external carotid artery before introducing the dilator. A 5 French (F) dilator is then placed over the microwire. The wire is removed, and a cervical angiographic run is performed (figure 1B). Under roadmap guidance, a 0.035 inch glidewire is introduced through the dilator, which is withdrawn into the common carotid artery, and the glidewire is advanced into the internal carotid artery (ICA) over which a 6 F sheath is placed directly into the ICA (figure 1C, D). The sheath is advanced as far distally as allowed by the tortuosity of the distal cervical ICA and secured to the skin in multiple places by sutures.
Main steps of direct carotid artery puncture access. (A) The common carotid artery is punctured with a 21 gauge micropuncture needle placed at a 45° angle, and a 0.018 inch soft tip microwire is advanced into the external carotid artery. (B) A 5 F dilator is placed over the microwire. Next, the microwire is removed and a cervical angiography run is performed. (C) Under roadmap guidance, the 5 F dilator is carefully withdrawn back into the common carotid artery just below the bifurcation, and a 0.035 inch guidewire is advanced into the ICA. (D) The dilator is removed, and a 6 F sheath is placed into the ICA. ECA, external carotid artery; ICA, internal carotid artery.
Placement of the 6 F sheath too close to the clavicle might result in sheath entry rather perpendicular to the course of the carotid artery, causing kinking of the sheath. A high carotid puncture (too close to the bifurcation) will result in the dilator entering either the external or internal carotid arteries. External carotid artery entry will require re-access. Therefore, ultrasound guidance is extremely useful to avoid access that is too proximal or too distal.
Once 6 F sheath access is established, systemic heparin is administered to achieve a therapeutic activated coagulation time between 250 and 300 s, and an intermediate catheter (such as the 5 MAX ACE or 5 MAX (Penumbra Inc, Alameda, California, USA), 058 Navien (ev3-Covidien, Irvine, California, USA), or 057 distal access catheter (Concentric Medical, Mountain View, California, USA)) is delivered into the distal ICA. From this point, various endovascular approaches, including the use of stent retrievers, direct aspiration (A Direct Aspiration First Pass Technique for the endovascular treatment of stroke (ADAPT)10), aspiration using the Penumbra system (Penumbra Inc), or other tools can be used, based on operator preference, local anatomic considerations, and location of the thrombus.
Closure
Manual compression of the puncture site is the most commonly utilized way to achieve hemostasis after sheath removal at the end of the procedure.7 ,11 ,12 This is typically done either immediately after the intervention with prior reversal of the effect of the heparin anticoagulation with protamine sulfate or by postponing sheath removal for several hours until the heparin effect wears off.
There is limited experience with the use of closure devices for carotid artery sheath removal in the current literature. Use of the Angio-Seal (St Jude Medical, St Paul, Minnesota, USA) was reported in six cases, without any complications, including cases of a large 8 F sheath requiring an 8 F Angio-Seal.12–14 This closure device works by delivering an absorbable intraluminal anchor attached to a collagen plug, which is left on the outer side of the artery.15
To our knowledge, the use of other closure devices in this specific location has not been described. In addition to applying manual pressure, we used a StarClose device (Abbott Vascular, Abbott Park, Illinois, USA) in two cases at the discretion of the attending neurointerventionist. The application of this device was uneventful in the first case, but a large neck hematoma developed in the second case (figure 2).
CT angiogram of the neck demonstrates a large intramuscular hematoma on the right side (arrows). This hematoma developed shortly after the use of a StarClose device (Abbott Vascular, Abbott Park, Illinois, USA) at the end of the procedure. The broken arrow points to the right common carotid artery. Additional manual pressure was applied for 40 min, and the hematoma remained stable. The patient had been intubated before the procedure and remained intubated for an additional 2 days, after which successful extubation was accomplished.
Illustrative cases
Case No 1: acute stroke (National Institutes of Health Stroke Scale (NIHSS) score of 14) caused by right middle cerebral artery M1 occlusion
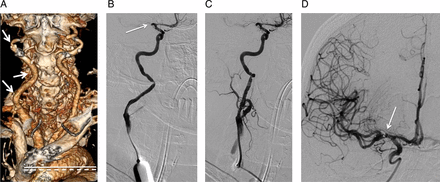
A type III arch and right common carotid artery tortuosity were observed on CT angiography; therefore, direct carotid access was chosen for this case (figure 3A). The microwire was passed through the micropuncture needle and a 5 F dilator was introduced. A cervical angiogram demonstrated that the microdilator was introduced into right ICA (figure 3B). A 6 F sheath was placed, and mild vasospasm was noted; however, the spasm was of no clinical significance (figure 3C). A 5 MAX ACE catheter was used for direct thrombus aspiration (ADAPT). Within 25 min following direct carotid puncture, Thrombolysis in Cerebral Infarction grade 3 reperfusion was achieved (figure 3D), and the patient subsequently made a dramatic neurological recovery.
(A) CT angiogram shows type III arch (broken line indicates level of the brachiocephalic artery origin; solid line shows inferior margin of the arch) and tortuosity of the right common carotid artery (arrows). (B) Cervical angiographic run, anteroposterior view, is performed through a 5 F dilator, confirming that the dilator tip is positioned within the cervical internal carotid artery. Proximal occlusion of the M1 segment of the right middle cerebral artery is seen (arrow). (C) Injection following placement of a 6 F sheath shows mild vasospasm of the cervical internal carotid artery around the sheath. (D) Final intracranial angiographic run through a 5 MAX ACE catheter (Penumbra Inc, Alameda, California, USA) (arrow points to catheter tip within the right M1 middle cerebral artery segment) shows robust filling of the entire right middle cerebral artery territory, representing Thrombolysis in Cerebral Infarction grade 3.
Case No 2: acute stroke (NIHSS 12) with unknown time of onset
Emergent CT angiography demonstrated right middle cerebral artery M1 occlusion and severe tortuosity of the proximal right common carotid artery (figure 4A). Direct carotid artery access was chosen in anticipation of challenging access due to the severe common carotid artery tortuosity. A 6 F sheath was placed too close to the clavicle, and because of the rather posterior course of the common carotid artery, the direction of sheath entry was almost perpendicular to the carotid artery. This caused severe kinking of the sheath (figure 4B) and made subsequent aspiration catheter delivery and aspiration thrombectomy less efficient (figure 4C, D). This case demonstrates the critical importance of selecting the appropriate level and angle for placement of the micropuncture needle, dilator, and sheath.
(A) Three-dimensional reconstruction of the CT angiogram demonstrates a very sharp angle of the right proximal common carotid artery (arrow). Notice the rather posterior course of the common carotid artery due to proximal kinking. (B) Anteroposterior (left) and lateral (right) angiographic views of a cervical injection through the 5 F dilator showing positioning of the dilator tip at the carotid bifurcation level (broken arrow). Notice the severe kinking of the proximal end of the dilator, best appreciated on the lateral view (arrows). (C) Injection through a 5 MAX ACE catheter demonstrates complete occlusion of the proximal right middle cerebral artery, consistent with Thrombolysis in Cerebral Infarction (TICI) grade 0. The arrow points to the location of the 5 MAX ACE catheter at the petrous carotid segment. (D) Direct aspiration of the clot via the 5 MAX ACE catheter resulted in only partial reperfusion of the affected territory (final TICI grade 2a), likely because of the decreased lumen of the 5 MAX ACE within the sheath and thus suboptimal aspiration power. Following withdrawal of the 5 MAX ACE at the end of the case, severe deformity of the catheter was appreciated.
Discussion
The initial cerebral angiography procedures in the early 1960s were performed exclusively through direct carotid or transbrachial access.16 As the neurointerventional field developed further and transfemoral catheters were introduced over the next decade, the transfemoral approach became the standard for cerebral angiography.
To this day, the transfemoral approach remains the standard technique in establishing access during neuroendovascular interventions, including intra-arterial stroke interventions. Recently, a case of successful ultrasound guided direct vertebral artery access in a patient with basilar artery stroke was reported.17 In this case, femoral and radial access approaches were attempted initially but were unsuccessful, and direct vertebral artery puncture was performed as a last resort.
Transbrachial or transradial access has been previously utilized for CAS and may be considered as another alternative approach for stroke interventions, especially in cases with a tortuous right carotid artery or bovine origin left carotid artery, where a challenging transfemoral route is anticipated.18 ,19 However, such access is technically difficult for catheterization of a non-bovine left ICA take-off (due to its sharp angle) and still does not solve the problem of ostial stenosis.20
For cases of CAS or aneurysm embolization, some operators advocate performing carotid artery puncture under direct roadmap guidance using a diagnostic catheter placed via a transfemoral route.7 ,12 ,13 Although such an approach minimizes the risk of carotid dissection and ensures accurate placement of the sheath at the target location, it might cause a delay in establishing successful reperfusion for cases of acute stroke interventions and have an adverse effect on outcomes. Instead, we rely on ultrasound imaging for rapid visualization of the carotid artery and bifurcation. Ultrasound navigation allows us to definitively evaluate the relationship of the jugular vein with the carotid artery, which is many times overlying the common carotid artery. It also allows rapid assessment of the bifurcation both in terms of level and disease.
Intravenous thrombolysis with tissue plasminogen activator is often administered prior to endovascular interventions. In such cases, extra caution should be taken when obtaining access and during access site closure. Although groin hematomas are mostly innocuous unless they extend into the retroperitoneal space, neck hematomas can result in an increased risk for cranial neuropathies, but most concerning is a potential for rapid airway compromise. Regarding patients taking antiplatelet agents, experience with direct carotid puncture for CAS did not show an increased risk of neck hematoma in patients on dual antiplatelet therapy; however, very few cases have been reported, thus limiting data interpretation.5 ,21 Similar to the population of post-intravenous thrombolysis patients, caution is advised in this population.
Currently, there is no vascular closure system to effectively address direct carotid percutaneous access. In our case with a postprocedure neck hematoma, the StarClose device entangled within the platysma muscle layer, preventing optimal apposition of the device to the vessel wall. This is likely to be similar for other closure devices. On the other hand, leaving the sheath in place for extended periods of time to await complete reversal of anticoagulation carries with it the risk of embolization from the sheath itself. We feel that reversal with protamine, with manual, non-occlusive pressure, may be our current best means to obtain hemostasis.
In a series of 27 patients with intracranial aneurysms treated via transcervical access, one case was complicated by formation of a focal neck hematoma associated with dyspnea 20 h after the procedure, requiring intubation.11 Dorfer et al7 reported their experience with direct carotid puncture for the treatment of aneurysms and arteriovenous malformations in five patients without any access related complications. Single case reports of patients who underwent CAS by direct percutaneous puncture also showed safety of the direct carotid approach.5 ,21
Transcervical access using a surgical cut-down approach allows direct arterial closure, theoretically decreasing the chance of hematoma formation.7 ,22 However, the longer duration of this more complex surgical access approach (in comparison with a simple percutaneous puncture which takes only a few minutes) will lead to delay in achieving recanalization, decreasing the chances for good neurologic recovery. Moreover, the cut-down approach is not suitable for strokes treated with intravenous thrombolysis, due to the high risk of soft tissue bleeding.
Presently, devices designed specifically for intracranial interventions via direct carotid access are not available. Such devices would ideally have a shorter length than conventional access catheters and microcatheters used with a transfemoral approach. This may have added benefits in terms of greater aspiration power applied from a shorter distance to the lesion and easier delivery of mechanical devices into the intracranial circulation. Most importantly, in cases with tortuous aortic and proximal carotid anatomy, direct carotid access is rapid and straightforward, greatly decreasing recanalization times and increasing effectiveness. Widespread availability of preprocedure proximal anatomy evaluation through CT angiography allows rapid screening for cases where transfemoral access may be difficult. We encourage neurointerventionists to consider a direct carotid artery puncture approach for patients with challenging anatomy as an alternative to traditional femoral access for stroke interventions.
Conclusions
Difficult anatomy, including unfavorable arch type, carotid tortuosity, or an ostial lesion, can make placement of the guide catheter into the common and internal carotid arteries challenging or even impossible. The main advantage of direct carotid artery access in stroke is the ability to bypass such anatomically unfavorable areas and significantly shorten the time from the beginning of establishing access to reperfusion. Caution is advised when establishing direct carotid artery access to avoid potential complications, including arterial dissection, hematoma formation, and sheath kinking.
Acknowledgments
The authors thank Paul H Dressel, BFA, for preparation of the illustrations, and Debra J Zimmer for editorial assistance.
References
Footnotes
-
Contributors Conception and design: MM. Acquisition of the data: MM and AHS. Analysis and interpretation of the data: all authors. Drafting the manuscript: MM. Critically revising the manuscript: all authors. Final approval of the manuscript: all authors.
-
Competing interests LNP receives grant/research support from Toshiba; serves as a consultant to Abbott, Boston Scientific, Cordis, Micrus, and Silk Road; holds financial interests in AccessClosure, Augmenix, Boston Scientific, Claret Medical, Endomation, Micrus, and Valor Medical; holds a board/trustee/officer position with Access Closure and Claret Medical; serves on Abbott Vascular's speakers’ bureau; and has received honoraria from Bard, Boston Scientific, Cleveland Clinic, Complete Conference Management, Cordis, Memorial Health Care System, and the Society for Cardiovascular Angiography and Interventions (SCAI). EIL receives research grant support, other research support (devices), and honoraria from Boston Scientific, and research support from Codman and Shurtleff Inc and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman and Shurtleff Inc; serves as a consultant per project and/or per hour for Codman and Shurtleff Inc, ev3/Covidien Vascular Therapies, and TheraSyn Sensors, Inc; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. EIL receives no consulting salary arrangements. All consulting is per project and/or per hour. MM has received an educational grant from Toshiba. AHS has received research grants from the National Institutes of Health (coinvestigator: NINDS 1R01NS064592-01A1) and the University at Buffalo (Research Development Award) (neither is related to the present submission); holds financial interests in Hotspur, Intratech Medical, StimSox, Valor Medical, and Blockade Medical; serves as a consultant to Codman and Shurtleff Inc, Concentric Medical, Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra Inc, Stryker Neurovascular, and Pulsar Vascular; belongs to the speakers’ bureaus of Codman and Shurtleff Inc and Genentech; serves on National Steering Committees for Penumbra Inc 3D Separator Trial and Covidien SWIFT PRIME Trial; serves on an advisory board for Codman and Shurtleff and Covidien Vascular Therapies; and has received honoraria from American Association of Neurological Surgeons’ courses, Annual Peripheral Angioplasty, and All That Jazz Course, Penumbra Inc, and from Abbott Vascular and Codman and Shurtleff Inc for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. AHS receives no consulting salary arrangements. All consulting is per project and/or per hour. KVS serves as a consultant and a member of the speakers’ bureau for Toshiba and has received honoraria from Toshiba. He serves as a member of the speakers’ bureau for and has received honoraria from ev3 and the Stroke Group.
-
Ethics approval Ethics approval was obtained from the University at Buffalo Health Sciences institutional review board (project No. 403427-3).
-
Provenance and peer review Not commissioned; externally peer reviewed.