Article Text
Abstract
Objective To describe the feasibility and safety of transradial access (TRA) in the interventional management of acute ischemic stroke (AIS).
Methods A retrospective review of the local institutional AIS interventional databases of three tertiary academic centers was performed and the use of TRA identified.
Results TRA was attempted in 15 (1.5%) of 1001 patients; it was used in 12 cases due to transfemoral access (TFA) failure and in 3 as the primary strategy. The mean age was 72.3±8.6 and 46% were male. Baseline National Institutes of Health Stroke Scale score was 19.5±8.7, two patients (14%) received intravenous tissue plasminogen activator, and mean time from last known normal to intra-arterial therapy was 17.0±20.1 h. Five patients had anterior circulation occlusive disease and 10 had vertebrobasilar occlusions. TRA was effective in allowing clot engagement in 13 of 15 cases: one patient had a hypoplastic radial artery that precluded sheath advancement and one had chronic innominate artery occlusion that could not be crossed. Mean time to switch from TFA to TRA was 1.9±1.3 h and the mean time from radial puncture to reperfusion was 2.2±1.0 h. Modified Thrombolysis In Cerebral Infarction 2b–3 reperfusion via TRA was achieved in 9 of 15 patients (60%). No radial puncture site complications were noted. At 90 days, two patients (13%) had a good clinical outcome and seven (50%) had died.
Conclusions Failure of TFA in the endovascular treatment of AIS is uncommon but leads to unacceptable delays in reperfusion and poor outcomes. Standardization of benchmarks for access switch could serve as a guide for neurointerventionalists. TRA is a valid approach for the endovascular treatment of AIS.
- Angiography
- Intervention
- Stroke
- Technique
Statistics from Altmetric.com
Introduction
Early reperfusion has been clearly associated with improved clinical outcome in large vessel occlusion acute ischemic stroke (AIS).1 ,2 Endovascular therapy has been increasingly performed in patients who fail or are not eligible for intravenous tissue plasminogen activator (IV tPA) with promising results. Despite advancements in technology, complex vascular anatomy may occasionally generate substantial delays in clot engagement in cases performed via transfemoral access (TFA). These challenging characteristics have been extensively described, and typically relate to aortic arch elongation and/or tortuosity, femoral/iliac and aortic atherosclerotic, or dissecting disease.3 The alternatives for neurointerventional procedures in patients with difficult anatomy include radial, ulnar, brachial, or axillary artery access, as well as direct carotid puncture.3–5 We aim to describe the feasibility and safety of transradial access (TRA) in the interventional management of AIS.
Materials and methods
We retrospective analyzed the local institutional interventional databases that included consecutive patients undergoing attempted intra-arterial therapy for AIS in three tertiary academic centers (Grady Memorial Hospital/Emory University from 2010 to 2014, Jackson Memorial Hospital/University of Miami from 2008 to 2012, and Long Island College Hospital from 2005 to 2012).
Patients in whom the TRA was used to approach the large vessel occlusion were included, regardless of whether TRA was used secondary to TFA failure or as the primary route. Demographic, radiologic and procedural features were recorded.
Transfemoral and transradial approach
The transfemoral approach encompassed different techniques. As a general rule, a short 8 or 9 Fr sheath was inserted and a balloon guide catheter was navigated into the craniocervical vessels over a long 5 Fr angled guide catheter or a Vitek (Thorocon NB; Cook, Bloomington, Indiana, USA). In cases of elongated arches, alternative techniques such as exchange techniques and different catheters and sheaths were attempted.
The transradial approach included a modified Allen test prior to puncture, consisting of pulse oximetry on the thumb followed by compression of both ulnar and radial arteries until the waveform and arterial oxygen saturation disappeared. Once the ulnar artery was released, the patient was considered to pass the test if there was normalization of waveforms and restoration of baseline saturation within 7 s. Pulse oximetry was performed throughout the entire case. A micropuncture kit (21 G) was systematically used for a puncture cephalad to the styloid process and this was followed by insertion of a microdilator. Ultrasound was used at the operator's discretion. A radial cocktail was typically used and its constitution varied according to institutional protocols, but often included intra-arterial infusion of a vasodilator (eg, nicardipine) prior to the insertion of the sheath. All patients had either a 6 Fr 80 cm sheath (Shuttle Introducer; Cook) or a short sheath with a distal access catheter (typically a 070 Neuron; Penumbra, Alameda, California, USA).
In the majority of cases an arterial compression device was used for radial artery hemostasis (TR Band; Terumo, Somerset, New Jersey, USA). The modified Thrombolysis In Cerebral Infarction (mTICI) scale was used for reperfusion grading.6 Good outcome was defined as modified Rankin Scale (mRS) score of ≤2 at 90 days. The results are shown as mean±SD (median).
Results
TRA was attempted in 15 (1.5%) of 1001 patients. At Grady/Emory, TRA was attempted in nine (1.4%) of 616 patients undergoing IAT for AIS, allowing clot engagement in seven. At Jackson/University of Miami, TRA was attempted in five (4.5%) of 110 patients undergoing IAT and was successful in four. Lastly, TRA was attempted and was successful in one (0.3%) out of 275 patients undergoing IAT at Long Island College Hospital.
The mean±SD age was 72.3±8.6 (median 73) and 46% were male (5 white patients, 6 black patients, and 4 Hispanic). They had a typical profile of vascular risk factors in patients with AIS treated with IAT: 66% were hypertensive, 36% were diabetic, and 42% were dyslipidemic. Baseline NIHSS score was 19.5±8.7 (median 20) and two (14%) received IV t-PA. Ten patients had vertebrobasilar occlusions and the remaining five had anterior circulation occlusive disease (one left middle cerebral artery (MCA) M1, one left MCA M2, one right internal carotid artery terminus (ICA-T), one right MCA M1, and one right cervical ICA). The mean±SD time from last known normal to IAT was 17.0±20.1 h (median 9.3 h).
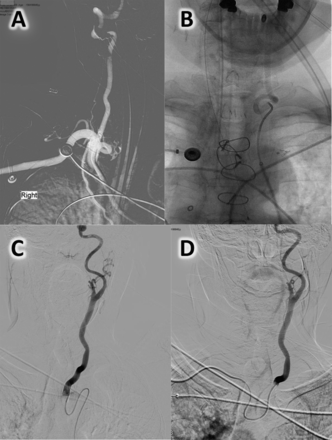
The TFA was used as a primary route in 12 of the 15 cases. One patient had severe iliofemoral atherosclerotic disease and significant subcutaneous fat deposits precluding sheath placement bilaterally (figure 1A). One patient had innominate occlusion at the site of a prior stent placement which obstructed direct access to the targeted right carotid system. The remaining 10 patients were found to have prominent aortic arch unfolding and tortuosity of the craniocervical large vessel origin and course that precluded sheath/catheter advancement (figure 2A, B) despite prolonged attempts with different sheaths, guide catheters, and wires. The TRA approach allowed navigation to the target lesion in all but two of these patients. The exceptions were one patient with substantial tortuosity and acute left common carotid artery origin and course in whom the procedure was terminated due to inability to reach an appropriate cervical position (figure 2C, D) and another with occlusion of the innominate artery that could be approached via TRA but could not be crossed.
Anatomic and pathological variations of access site. (A) Right external iliac atherosclerotic occlusive disease precluding femoral sheath navigation. (B) Left radial artery anteroposterior angiogram showing a hypoplastic radial course that precluded advancement of a hydrophilic-coated 6 Fr sheath into the brachial artery.
Illustrative cases of tortuous aortic arches and unfavorable craniocervical artery origin angles. (A) Right subclavian artery anteroposterior (AP) roadmap from transfemoral access (TFA) access showing a 360° vertebral artery proximal loop. (B) Unsubtracted AP left vertebral artery angiogram via TFA depicting prominent tortuosity of the proximal vertebral artery course. (C) Left common carotid artery AP angiogram from TFA showing a type III arch (note the course of the catheter). (D) Left common carotid artery angiogram (C) from right transradial access in the same patient indicating the unfavorable anatomy; access to the carotid of this patient was not possible.
Owing to expected tortuosity from non-invasive imaging (n=2) and aortic arch dissection (n=1), three of the 15 patients underwent TRA as a primary strategy. In two patients this was an effective approach but in the other the 6 Fr sheath could not be advanced due to the presence of a hypoplastic radial artery (figure 1B). Although TFA was used appropriately and full reperfusion was achieved, this patient was considered a TRA failure (no reperfusion via radial access).
The overall procedural length was 3.8±1.5 h (median 3.7 h). In the cases in which access had to be switched, the mean time interval from TFA to TRA was 1.9±1.3 h (median 1.4 h). In the cases in whom TRA was used, the mean time from radial puncture to reperfusion was 2.2±1.0 h (median 2.2 h). Intra-arterial thrombolytic agents were infused in 40.0% of patients, Merci retriever was used in 28.5%, thromboaspiration in 46.0%, and stent-retrievers in 33.3% of cases. The rate of mTICI 2b–3 reperfusion via TRA was 60% (9/15). No radial puncture site complications were noted. At 90 days two patients (13%) had a good clinical outcome and seven (50%) had died.
Discussion
Unfavorable iliofemoral and aortic anatomy may lead to extensive delays in clot engagement during interventional management of AIS. We demonstrate that TRA is a valid and feasible alternative in this setting.
The TFA has been primarily used by neurointerventionists due to the adequate size for large diameter catheterization and relatively low puncture-related complications. Despite significant advances in hardware technology and procedural techniques, groin characteristics such as severe atherosclerotic disease in iliofemoral arteries and prominent groin fat deposits may lead to significant difficulties in safe and effective sheath placement. Abnormalities in the aorta such as dissections and aneurysms, unfolding or unfavorable aortic arch (eg, type III arch), tortuous craniocervical vessel origin and course may also contribute to extreme difficulties to cerebral artery catheterization.7 Ribo et al7 reported that 5% of 130 patients who underwent IAT via TFA could not have the carotid artery catheterized, and these patients had lower recanalization rates and worse outcomes. This study corroborates the notion that this problem is not rare and affects outcomes.
The TRA in coronary angiography was introduced in the 1980s.8 The advantages of this approach include reducing puncture site complications and post-procedure bed rest time, and increasing patient comfort.9 Radial access in neuroangiography was described approximately a decade later in diagnostic cerebral angiography and carotid stenting, and more recently in aneurysm coiling.10–12 Recent cardiac literature has demonstrated a potential benefit of TRA over TFA in percutaneous interventions for acute coronary syndrome (lower risk of death and myocardial infarction).13 ,14 This advantage may relate to fewer access site complications (large hematomas and pseudoaneurysms).15 Although the clinical benefit in coronary patients remains controversial, it is fair to state that the utilization of TRA for emergency cases in the setting of heavy antithrombotic use has been validated and proved to be effective and safe.
Catheterization through TRA provides a less invasive and safe alternative, with access to all relevant brachiocephalic arteries for mechanical thrombectomy.16 It is important to appreciate that TRA has a significant learning curve and is said to require commitment prior to obtaining its rewards.3 The vertebral arteries should be approached through an ipsilateral TRA approach. Considering the typical degree of tortuosity of the arterial system of patients with AIS, the right and left carotid arteries can be accessed via right TRA (especially in individuals with type II–III aortic arch configuration).3 Cerebral thrombectomy normally requires a relatively high catheter/sheath placement and is therefore not ideal for patients with significant proximal/ostial common carotid artery disease.3 This does not necessarily apply to cases with vertebrobasilar disease since the angle for catheterization is much less problematic.
Although the inability to catheterize craniocervical vessels via TFA was uncommon in our endovascular cohort, prominent delays to reperfusion were noted in these cases. This translated into poor functional outcomes and a high mortality rate. The decision to switch from the femoral to the radial approach took nearly 2 h. Moreover, the time from radial access to the end of the procedure was high, potentially indicating that arterial tortuosity may lead to difficulties irrespective of access site and/or that neurointerventionalists are not as proficient with TRA as with TFA (due to the learning curve associated with this access and its infrequent utilization in neuroendovascular cases). A recent review recommended the development of protocols for when to use alternative access sites (eg, radial, brachial, carotid).17 Ribo et al have defined ‘difficult access’ as cases in which the time from groin puncture to carotid (common or internal) catheterization was >30 min. This cut-off was based on patients who had times to catheterization longer than the fourth quartile within their institutional cohort.7 Benchmarks based on this criteria derived from larger samples would be useful as a guide for when to switch, and this could have a positive impact on learning curve, procedural time and, potentially, clinical outcome.
Conclusion
The TRA to mechanical embolectomy is a valid approach for the endovascular treatment of AIS. Failure of TFA in the endovascular treatment of AIS is uncommon but leads to unacceptable delays in reperfusion and poor outcomes. Standardization of benchmarks for access switch could serve as a guide to neurointerventionalists.
References
Footnotes
Contributors DCH: design of the work, data acquisition, interpretation of data, drafting of the manuscript. RGN, KGD,RNP, NJ, ECP, MSE: data acquisition, critical revision of manuscript. KR: critical revision of manuscript. DRY: study conception, data acquisition, interpretation of data, critical revision of manuscript. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests RGN has potential conflicts with Stryker Neurovacular (Trevo-2 Trial PI, DAWN Trial PI), Covidien (SWIFT and SWIFT-PRIME Steering Committee, STAR Trial Core Lab) and Penumbra (3-D Separator Trial Executive Committee). DRY is a consultant to Boston Scientific, Micrus, Abbott Vascular, and Coaxia, and has received travel support from Abbott Vascular.
Ethics approval Ethical approval was obtained from the local Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The unpublished data from this dataset is held by Jackson Memorial Hospital/University of Miami School of Medicine and DRY as well as Grady Memorial Hospital/Emory University and RGN. Requests for data sharing would be required to be discussed with them directly.