Article Text
Abstract
Background Adequate dual antiplatelet (AP) therapy is imperative when performing neurovascular stenting procedures. Currently, no consensus for the ideal AP regimen exists. Thus the present study aimed to gain a better understanding of real world practice AP patterns by surveying neurointerventional surgeons.
Methods Survey links were emailed to 296 neurointerventional surgeons practicing in the USA, asking 51 questions including demographics, stent specific use, AP pre and post-medication, types of APs, point of care (POC) assessment, complications, and outcomes. Data were collected and analyzed using Research Electronic Data Capture (REDCap).
Results 74 participants responded; 56.8% were from academic centers. Participants treated an average of 5.5 aneurysms per month. They placed an average of 1.6 intracranial stents and 1.4 cervical stents per month. Mean number of pipeline embolization devices (PEDs) placed per year was 15.2. Heterogeneity existed regarding AP regimens; the most frequent included acetylsalicylic acid (ASA) 325 mg+Plavix 75 mg daily (for 7 days prior) and ASA 325 mg+Plavix 75 mg daily (for 5 days prior) for routine placement of intracranial and cervical stents, respectively. For emergency placement, ASA 325 mg+Plavix 600 mg (at time of surgery) was the most frequently used. 46.8% routinely used POC testing, most frequently VerifyNow (Accumetrics, San Diego, California, USA); the most common threshold determining a non-responder was <30% inhibition. 85.7% used POC for PED placement. Management changes based on POC testing were diverse.
Conclusions The results highlight the heterogeneity of current practices regarding AP medication regimens during neurovascular stenting. Given its importance, evidence based protocols are imperative. Minimal literature exists focusing on neurovasculature, and therefore understanding current practice patterns represents a first step toward generating these protocols.
- Stent
- Platelets
- Standards
- Flow Diverter
- Drug
Statistics from Altmetric.com
Introduction
The evolution of neurointerventional surgery has been characterized by the development and introduction into clinical practice of increasing numbers of devices that have expanded the indications and increased success rates of neuroendovascular treatment. For example, stents are increasingly utilized in neuroendovascular procedures for indications such as carotid stenosis, aneurysm embolization with coils, and severe refractory intracranial stenosis. Additionally, new flow diversion devices that serve as an endothelial scaffold and consist of densely woven metallic mesh material are recently being utilized for aneurysm obliteration.1–5 Because these devices are inherently thrombogenic, appropriate medical management in the periprocedural period is essential to minimize complications. Currently, the use of antiplatelet (AP) therapy is considered an essential requirement in the setting of neurovascular stent and flow diverter treatment.6–8
There is no broadly accepted consensus for the ideal AP regimen. A standard protocol is difficult to determine because there are no dedicated studies to establish doses, duration, and combination of medications, and the number of AP medications is continually increasing. Additionally, individual patient responses to certain AP medications have been found to vary significantly. This has propelled the development and utilization of point of care (POC) platelet reactivity testing in many centers. As a result, current practices surrounding AP regimens appear to be very heterogeneous and often based on training centers and regional trends, rather than scientifically obtained data.
Given the importance of adequate AP medication when performing neuroendovascular stenting procedures, evidence based protocols are imperative. As there are no randomized studies focusing on neurovasculature on which a high level of certainty can be based, understanding current practice patterns represents a first step toward generating these protocols. As such, a survey of US neurointerventional surgeons’ use of periprocedural AP medications was performed. The present study reports the survey's results regarding the routine practice of AP agents when placing neurovascular stents and flow diverters.
Methods
In February 2013, a cover letter and survey link were emailed to 296 neurointerventional surgeons practicing in continental USA, Puerto Rico, and Hawaii (excluding Alaska as no permanent, non-traveling neurointerventional surgeon could be identified). Individuals and email addresses were preliminarily referenced for identification by the American Association of Neurological Surgeons/CNS Cerebrovascular Joint Section website, and the Society of Neurointerventional Surgery membership roster from the 8th Annual Joint Meeting published online. A web search was performed to identify the major academic and private institutions and the associated interventional neuroradiologists. Practitioners, their affiliated institution, and email addresses were confirmed to be current and responsive to email.
Using the Research Electronic Data Capture (REDCap) application (REDCap Software, V.5.2.1, 2013 Vanderbilt University), a survey (see supplementary appendix A, available online only) was composed.9 At least two surveys were mailed per state. Wherever possible, neurointerventional surgeons practicing in larger population areas, as well as within different cities, were selected (judgment sampling); otherwise, the selection was random. However, using this methodology, multiple neurointerventional surgeons in the same practice and/or institution received the survey. Moreover, each physician listed his or her practice, thereby allowing assessment of inter-institutional variability. Survey responses were deidentified as participants’ names and email addresses were removed from responses. Three reminder emails were sent to increase the survey response rate.
The survey sought responses to 51 primary questions, excluding secondary branching logic, pertaining to various aspects of the practice of AP therapy as it applies to neurovascular stenting, including demographics, specific stent use, premedication with AP agents prior to stenting, maintenance medication with AP agents after stenting, types of AP medications prescribed, POC assessment of platelet aggregation assay testing, routine and emergency intraprocedural and postprocedural complications, and outcomes. The name of the responding institution or practice, specialty/subspecialty, degree, title, institutional affiliation, years in practice, average aneurysms, intracranial stents, and cervical stents the individual and department treated per month were recorded at the beginning of the survey. X2 tests were conducted to compare AP therapy utilized at academic and non-academic centers. Frequencies were calculated based on number of participants responding to a particular question.
Results
Demographics
Of the 296 neurointerventional surgeons surveyed, 74 (25.0%) responded. The mean number of years practicing independently was 12.6±8.1 years. Of the respondents completing the surveys, 42 were academic (56.8%), 11 private (14.9%), 18 mixed academic/private (24.3%), and three responded to other (military, hospital based, academic/multispecialty group practice) (4.1%). Responses were received from seven (9.5%) women. The specialty/subspecialist distribution is shown in figure 1.
Number of respondents who identified themselves as being part of a particular specialty. Each respondent could identify multiple specialties.
Regarding practice volume, the mean numbers of aneurysms treated monthly, per individual and department, were 5.5±3.5 and 12.1±17.8, respectively. The mean numbers of intracranial stents placed/used monthly, per individual and department, were 1.6±1.4 and 3.5±5.4, respectively. The mean numbers of carotid cervical stents placed/used monthly, per individual and department, were 1.4±1.3 and 2.5±2.4, respectively. The types of cerebrovascular stents used by each institution are listed in table 1. The mean number of pipeline embolization devices (PEDs) (eV3/Covidien) used per institution per year was 15.2±16.0.
Cerebrovascular stents used per institution
Types of AP medications, frequency of use, and regimens
Specific AP medications ever and routinely used (>80% of the time) to prevent or treat thrombotic complications are listed in table 2. The three most commonly used medications were clopidogrel (93.8%), aspirin 325 mg (81.5%), and aspirin 81 mg (32.3%).
Antiplatelet medications used to prevent or treat thrombotic complications in cerebrovascular stent patients
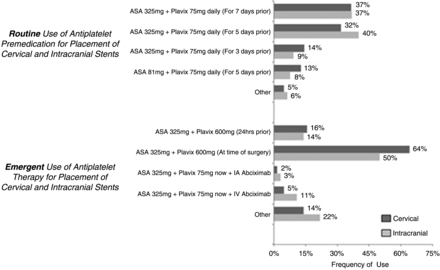
Regarding the practice of premedication with AP agents prior to routine placement of a cervical or intracranial stent, the most common regimen was acetylsalicylic acid (ASA) 325 mg+Plavix 75 mg daily (for 7 days prior) (36.5%) and ASA 325 mg+Plavix 75 mg daily (for 5 days prior) (40%), respectively. For emergency placement of both cervical and intracranial stents, the most common practice was ASA 325 mg+Plavix 600 mg (at time of surgery) (64.1% and 50%, respectively) (figure 2). There were multiple other regimens reported, which are described in supplementary appendix B (available online).
Percentage of respondents who utilized a particular antiplatelet regimen prior to routine and emergency placement of cervical and intracranial stents. ASA, acetylsalicylic acid.
Maintenance AP medication regimens after placement of cerebrovascular stents are depicted in figure 3. The most common practices for cervical and intracranial stents were ‘other’ and ASA 325 mg daily for life+Plavix 75 mg daily for 3 months, respectively. The other regimens reported by participants are listed in supplementary appendix C (available online). When stopping an AP agent, nine (13.8%) of the respondents tapered the medication prior to discontinuing.
Percentage of respondents who utilized a particular antiplatelet regimen after placement of cervical or intracranial stents. ASA, acetylsalicylic acid.
Point of care testing for platelet reactivity—frequency, thresholds, and management changes
Regarding the use of POC testing for platelet reactivity, 47 (72.3%) participants responded ‘yes’ to the question, “Does your department use POC platelet aggregation assays such as VerifyNow or P2Y12 to determine the minimal AP dose to achieve inhibition or resistance to a particular AP agent?”, 16 (24.6%) responded ‘no’ and two (3.1%) responded ‘I don't know’. Of those who responded ‘yes’, additional information pertaining to type and frequency of POC assessment was ascertained (table 3). The most common type of POC test utilized was the VerifyNow P2Y12 Assay (Accumetrics, San Diego, California, USA) (36, 76.6%), and the most common threshold for determining a non-responder was <30% inhibition (11, 23.9%) (table 4).
Specific point of care assessments utilized
Institutional non-responder Plavix cut-off values
Of those who reported utilization of POC testing for platelet reactivity, the frequency of use was assessed: 22 (46.8%) responded ‘always’, 21 (44.7%) responded ‘sometimes’, and four (8.5%) responded ‘I don't know’. Of those who did not always use POC testing, the majority responded that it was not necessary, not standard of care, or that there were limited data supporting its use. For participants who ‘sometimes’ utilized POC testing, the majority used it for PEDs (18, 85.7%). One participant each used it for ‘elective stent cases’, ‘small diameter vessels’, and ‘patient history’. Of the 16 (24.6%) who responded ‘no’ to utilizing POC testing, the described reasons were: ‘using platelet aggregation studies to test for aspirin or Plavix resistance’ (1, 6.3%), ‘testing is not inhouse’ (1, 6.3%), ‘expense and time’ (3, 18.8%), ‘clinical relevance’ (3, 18.8%), ‘use of alternative testing (platelet mapping, thromboelastography, or light transmittance aggregometry)’ (1, 6.3%), ‘hematologists and laboratory do not support it’ (2, 12.5%), ‘high false negative rates’ (1, 6.3%), ‘unclear interpretation of results’ (2, 12.5%), ‘in process of purchasing VerifyNow Unit’ (1, 6.3%), and one did not report a reason (1, 6.3%).
Participants were asked what specific management changes they would make based on identifying a Plavix non-responder through POC testing. The answers were extremely heterogeneous and are listed in table 5. The most common answer was to ‘double the dose’ (22, 43.1%). Participants were also asked if their maintenance AP practice was different after placing a flow diversion device. Of the 65 participants who responded to this question, 26 (40%) responded ‘no’ and 39 (60%) responded ‘yes’. The specific answers to ‘yes’ responses are listed in supplementary appendix D (available online). The answers were diverse, but the most common was ‘ASA 325 for life and Plavix 75 for 6 months’.
Changes to medical management for Plavix non-responders, as determined by point of care testing
Hemorrhagic and thrombotic complications
Regarding intraprocedural complications, the majority of participants answered that ‘less than 1 in 20’ patients experienced in-stent thrombosis or hemorrhage during a routine or emergency intracranial or cervical stent procedure. Higher rates of both thrombotic and hemorrhagic complications were reported for intracranial than for cervical stents, especially during emergency procedures (table 6). The most common rate of postprocedural thrombotic and hemorrhagic complications for routine and emergency intracranial and cervical stent placement was also ‘less than 1 in 20’ (table 7). Regarding typical timing of postprocedural hemorrhage following intracranial stenting, 44% selected ‘1–7 days’ and 38% selected within the ‘first 24 h’.
Intraprocedural complications related to neurovascular stenting
Postprocedural complications related to neurovascular stenting
Discussion
In order to reduce thrombotic complications associated with placement of a neurovascular stent, use of dual AP medications has become routine practice. Although most protocols are derived from the cardiac literature, there is extreme heterogeneity among the medication regimens utilized while performing neurovascular stent procedures, including type of drug, dose, duration of treatment, and use of POC platelet reactivity testing. The present study was performed to assess and categorize these heterogeneous practice patterns, as a first step toward building future clinical trials and emphasizing the need for evidence based guidelines.
Adequate dual AP medications appear necessary prior to placement of a neurovascular stent. In the literature, rates of ischemic events associated with stent assisted coil embolization are reported to vary between 2% and 21%, and have been found to increase when dual AP medications are incorrectly loaded or are stopped prematurely.10–13 Conversely, inappropriate use of AP medication may also contribute to hemorrhagic complications. Hemorrhage rates while utilizing dual AP medications after placement of neurovascular stents have been reported as between 1% and 4% for carotid artery stenting and approximately 6% for intracranial aneurysms undergoing stent assisted coil embolization.6–8
Albeit self-reported, the most common rates of thrombosis and hemorrhage described in the current survey were <5%, but were increased for intracranial and emergency stenting procedures. This finding is consistent with the literature.6 ,14 As intracranial stenting procedures require use of more distal catheters, are often longer in duration, and do not include distal embolic protection, the likelihood of thrombus formation or ischemia is increased, potentially contributing to this difference. Additionally, premedication with dual AP medications may be inadequate during emergency stenting procedures. This may lead to thrombosis requiring intra-arterial or intravenous AP agents, such as abciximab. These ‘rescue’ AP medication protocols have been associated with increased hemorrhage rates.15 Furthermore, multiple studies have demonstrated increased complication and hemorrhage rates when placing intracranial stents in patients with acute subarachnoid hemorrhage.6 ,16 ,17 The present survey results emphasize the need to focus on determining the safest and most effective AP regimens for intracranial and emergency stenting procedures.
Standard practices regarding AP medications for stenting are better defined in the cardiac literature, and most stem from type B level of evidence. The 2011 guidelines from the American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions for percutaneous coronary intervention describe recommendations for dual AP medication surrounding the placement of coronary stents. Loading patients with ASA and a P2Y12 inhibitor such as clopidogrel, prasugrel (in patients without a stroke and <76 years of age), or ticagrelor are recommended. Additionally, taking ASA indefinitely and a P2Y12 inhibitor for a minimum of 1 month, or longer when placing drug eluding stents or for patients with acute coronary syndrome, is suggested for maintenance therapy.18 Although these guidelines are derived from some prospective studies with relatively large sample sizes, the subjects are cardiac patients receiving coronary stents. Caution must be exercised when applying these guidelines to the neurovascular population, as placing a stent in the cerebrovascular circulation may be inherently different given differences in vascular anatomy, consequences of hemorrhage, and exposed surface area of stents.19 ,20
Regarding AP medication recommendations for neurovascular stenting, there are currently few formal published guidelines. Existing studies specific to neurointerventional procedures consist mainly of registries, retrospective case series, or expert opinion, all type C level of evidence.11 ,12 ,21–23 In addition to extrapolating from the cardiac literature, practices tend to be regionally or locally based regarding pre-, maintenance, and POC testing surrounding AP medications for neurointerventional procedures. The current survey confirms this extreme heterogeneity, a pattern that makes future controlled trials difficult to design.
One reason for this heterogeneity includes the rapid introduction of newer AP agents, such as Prasugrel, and controversy surrounding the utility and efficacy of POC platelet reactivity testing, such as Verify Now. In 2010, the Food and Drug Administration issued a black box warning that clopidogrel was less effective in certain patients with genetic differences in CYP2C19 function.24 In response, prasugrel, a third generation P2Y12 inhibitor, was developed. This drug has a faster onset and is effective despite these genetic variations or concurrent treatment with proton pump inhibitors, making it potentially superior to clopidogrel.19 Conversely, recent studies caution that it may be associated with higher hemorrhage rates, making routine use of this medication controversial.25 ,26 In the present study, 10.8% of respondents routinely used prasugrel while 46.2% reported having ever used this medication. Additionally, 11 of 51 participants stated that they would change clopidogrel to prasugrel based on POC testing demonstrating a ‘non-responder.’ These results further demonstrate the need for future trials assessing the relative safety and efficacy of next generation AP medications.
Regarding POC platelet reactivity testing, significant controversy continues regarding indications, reliability, thresholds, and management changes based on results. The majority of the existing literature involves cardiac patients and reports varying results. A recent meta-analysis of 4213 coronary patients in 10 prospective randomized trials reported improved outcomes without significant increases in hemorrhage events when AP therapy was augmented based on POC results. This was especially true for patients at high risk for stent thrombosis.27 There is limited neurovascular literature and most is retrospective, single center, case series.26 ,28 Delgado et al found increased thromboembolic and hemorrhagic complication rates in patients undergoing PED placement with P2Y12 reaction units (PRUs) >240 and <60, prompting them to create a complex algorithm of AP modifications tailored to POC results. Their findings are limited given a sample size of 44 patients and no patients with a PRU range of 201–240.29 The present study demonstrated wide variability regarding utilization of POC testing and management based on the results; 72% of respondents utilized POC testing and the most common type was VerifyNow; 45% utilized POC sometimes, the majority of which was for flow diverter cases. The most common threshold for a non-responder was a value of <30% inhibition. Interestingly, as of 2012, the VerifyNow test no longer reports per cent inhibition, but only a PRU value. These results again emphasize a diversity of practice patterns and the potential need for standardization.
Concurrent with the introduction of newer medications and testing, novel endovascular devices such as flow diverters have been recently approved and incorporated into routine practice. Although they provide a vital treatment alternative for specific aneurysms, these devices are thought to be more thrombogenic and associated with delayed intracranial hemorrhages, making management of AP medications more important.5 ,30 ,31 In our present survey, 60% of respondents changed their AP practices when placing a flow diverter, as opposed to a cervical or intracranial stents. Descriptions of altered practices were very variable but the most frequent change in practice was to continue Plavix until the aneurysm is obliterated, reported by 15.4% of respondents. Additionally, many respondents stated that placing a flow diverter was an indication for utilizing POC testing. Overall, given the increased frequency of hemorrhagic and thrombotic events associated with flow diversion devices and the increased variability of AP medication practices when performing this treatment, further study and guidelines are needed to improve patient outcomes.
The present survey has several limitations. Answers to questions of procedural volume and event rates were self-reported. This may not be as accurate as objectively obtained data. Additionally, effort was made to include a diverse group of neuroendovascular surgeons; however, the highest response rate was from those in an academic setting, which may contribute to a response bias. Finally, response rate was low, which may limit the ability to generalize these results.
Moreover, although we collected responses for AP practice in both cervical and intracranial procedures, it is possible, or even likely, that what will ultimately be determined to be the best practice may differ between these two types of procedures as well as between stents and flow diverters.
Conclusion
Overall, responses from this survey highlight the heterogeneity of current practices regarding AP medication regimens during neurointerventional procedures. Although management is guided by the cardiac literature, which may not be generalizable to the neurovascular population, it is extremely diverse and dictated by regional and local practices. Additionally, minimal literature exists regarding AP medication regimens specifically for neurointerventional procedures. Consequently, establishing future trials that directly compare practices are challenging given the lack of an industry standard. This highlights the need for guidelines based on available literature and expert consensus.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors All authors contributed equally to this work. RWFF and MJS conducted the survey composition and distribution. All authors contributed to the main manuscript composition, revisions, and edits. All authors discussed the results and implications, and commented on the manuscript at all stages.
-
Competing interests None.
-
Ethics approval The study was approved by the institutional review board of the University of Pennsylvania.
-
Provenance and peer review Not commissioned; externally peer reviewed.