Article Text
Abstract
Background Flow diverter stents (FDS) have been described as a breakthrough in the treatment of intracranial aneurysms. Of the various flow diverter models, the Pipeline device has been the main approved and used device, with established and good long-term results.
Objective To present the first series of patients treated with its new version, the Pipeline Flex device. This has kept the same device design and configuration but redesigned and completely modified the delivery system.
Methods In this technical report, we include 10 consecutive patients harboring 12 saccular aneurysms of the anterior circulation. We report the main changes on the system, immediate results, and technical nuances with illustrative cases.
Results We implanted 12 devices, including 11 Pipeline Flex and one Pipeline device. We used the old version in one case that required a second layer with a short length not available in the Pipeline Flex size range. All attempts at treatment were successful and no device was discharged or removed. Recovery was required or used in half of the cases with good or excellent performance, except in one case that presented with multiple proximal loops and tight curves. We had two transitory events without ischemic lesions on MRI that recovered 1 and 4 h after all patients were discharged home asymptomatic.
Conclusions Pipeline Flex represents a major advance in FDS technology. The redesigned system has significantly improved the deployment of the Pipeline stent, by enabling the operator to resheath the device. It has the potential to continue revolutionizing the endovascular approach for intracranial aneurysms.
- Aneurysm
- Flow Diverter
- Stent
- Technique
- Technology
Statistics from Altmetric.com
Introduction
Flow diverting stents (FDS) have been reported as a major breakthrough in the endovascular therapeutic field.1–6 Of the different FDS systems, the Pipeline device (Covidien Neurovascular, Irvine, USA) is one of the most widely used and has been approved by most national regulatory agencies worldwide. Recently, published data have demonstrated its safety and effectiveness for treatment of anterior circulation saccular intracranial aneurysms (IAs).1 ,5 ,6 The first-generation Pipeline stent demonstrated consistency on deployment (99% of attempts in the PUFS study) and good occlusion rates at 6 months (82%).1 The device has been described as suitable for treatment of large neck and complex IAs; however, its deployment requires training and has some drawbacks in comparison with regular intracranial stents or other flow diverters. Resheathing of the capture coil that permits the navigability of the devices through the microcatheter can pose a challenge in cases requiring a telescoping procedure in small and tortuous arteries. Proximal migration of the Pipeline device can occur and since it cannot be resheathed, repositioning of this flow diverter is not possible.
The new Pipeline Flex device has a completely redesigned delivery system that can overcome these problems but still keeping the same stent design, material and configuration. The capture coil has been removed and substituted for double Teflon leaves to facilitate navigation. The device is now almost completely resheathable, with a marker indicating the point of no return for recapture. In addition, the pusher wire has been replaced by a larger, elongated anti-tube to permit better pushability. We report in this paper the initial experience with this new device and some technical nuances in a consecutive series of 10 patients with 12 saccular IAs.
Methods
We included prospectively and consecutively all patients treated with the new Pipeline Flex device. We selected patients harboring saccular IAs located in the anterior circulation. We excluded intracranial dissections, previously treated, or fusiform aneurysms. The radiological, epidemiological and clinical data were prospectively collected at our aneurysm clinic and perioperative images and clinical information were externally adjudicated (MK, TK). The procedures were performed by two physicians experienced with the Pipeline stent (VMP, PV). Clinical examinations were performed on admission, during hospitalization, and at discharge (GE, AP, EM, HY). The management of IAs in our institution consists of a multidisciplinary evaluation by the neurosurgical and neuroradiological teams, and only those patients who were deemed eligible for FDS treatment were included.
Procedure and center guidelines
Patients were prepared with double antiaggregation (clopidogrel 75 mg and aspirin 100 mg) 10 days before the procedure. The evening before the procedure an aggregation inhibition test was performed to ensure a minimum of 40% aggregation inhibition; this is performed with the VerifyNow test for a FDS procedure in our institution. When inhibition was incomplete, a loading dose of 600 mg Plavix was given and a new test was performed. The procedure was carried out only if the extent of antiaggregation met the institutions’ protocol.
Endovascular procedure and strategy
An 8F femoral sheath was the standard approach for endovascular cases. An 8F-guiding catheter (Penumbra, Alameda, California, USA) was selectively placed into the corresponding common carotid artery. A distal flexible catheter Navien 5 or 6F (Covidien Neurovascular, Irvine, California, USA) was placed selectively into the internal carotid artery (ICA) at the level of the petrous segment or distal cervical portion. Then, a Marksman microcatheter (Covidien, Irvine, California, USA) was navigated distal to the IAs at the level of the middle cerebral artery through a 14 μm guidewire. For most cases, we planned one stent layer without associated coiling unless a subjective risk of rupture based on the patient’s history was found. Clinical and imaging (MR angiography and angiography) follow-up is planned at 6, 12, and 24 months according to our local protocol.
Pipeline Flex device
The Pipeline Flex is the newest evolution of the first Pipeline device (figure 1). The new device has kept the same stent design and structure (75% chromium–cobalt/25% platinum–tungsten), with 48 strands interwoven in a standard pattern, but the delivery system has been completely redesigned (figure 1). The stent itself is mounted over a stainless steel delivery wire. The distal part of the stent is covered by two 3 mm protective sleeves of polytetrafluoroethylene, replacing the capture coil of the old version. The distal tip is now a soft hydrophilic 0.012″ wire with a tip angle of 55° and a proximal platinum marker for visibility. The proximal part of the stent lies on a resheathing pad that is a silicone elastomer with a profile of 0.023″ between two platinum markers, of which the most anterior one is the resheathing marker. The pusher wire is a hypotube which is thicker and longer than that of the previous version.
New Pipeline Flex device. (A) Schematic drawing showing the new device with a magnification of the proximal end of the device and the resheathing pad: (1) tip coil, (2) proximal bumper, (3) introducer sheath, (4) stent, (5) fluorosafe marker, (6) delivery wire, (7) distal marker, (8) PTFE sleeves, (9) resheathing pad, (10) resheathing marker. (B) Fluoroscopic view of the device undeployed. PTFE, polytetrafluoroethylene.
Technical nuances
The Pipeline Flex device is a self-expandable device with high radial force without the distal anchoring found in the first version (figures 2⇓–4). It opens as it is being pushed out of the Marksman microcatheter and the distal end of the stent is fully opened once the first 10–15 mm of the device are deployed. Thus, two strategies can be used to begin the deployment: (1) the initial 10 mm are deployed distal to the target lesion (eg, distal middle cerebral artery) and, the partially deployed device is subsequently withdrawn to the planned landing zone (eg, the ICA); (2) the device is navigated to the landing zone and the microcatheter is unsheathed progressively until it is deployed. The first technique is usually used when there is a short artery length on the landing zone or when there are tight curves proximally. The second technique is used for proximal lesions when the system support is sufficiently stable to permit microcatheter unsheathing with small risk of sudden descent of the whole system.
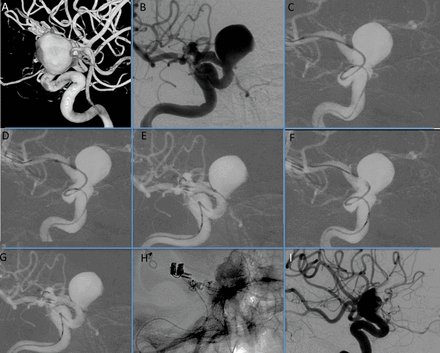
Patient 1 presenting with recent headaches and family history of subarachnoid hemorrhage. (A) 3DRA-XperCT view showing two aneurysms at the level of the ophthalmic segment of the internal carotid artery (ICA: arrow) and on the origin of the ophthalmic artery (arrowhead). (B) Left ICA anteroposterior roadmap from DSA showing one of the initial maneuvers of deployment: pushing out the device at the M1 level. Note the distal wire with a smooth curve (double arrows) and the stent partially opened (double arrowheads). (C) Left ICA lateral roadmap from DSA showing the retraction of the system (microcatheter and stent combined) to the desired landing position, proximal to the anterior choroid artery in this case. (D) Left ICA lateral roadmap from DSA showing a proximal migration of the system. (E and F) Left ICA roadmap from DSA showing recapturing maneuver to reposition the device on the planned landing zone. (G and H) Non-subtracted DSA lateral position showing the device partially and completely deployed as well as the resheathing marker (red arrow). (I) Non-substrated DSA series showing the final control after stent deployment and the final apposition.
Patient 4 presenting with recent headaches. (A) 3DRA showing the aneurysm located at the ophthalmic segment of the internal carotid artery (ICA). (B) DSA on anteroposterior (AP) view showing the preoperative angiogram. (C, D, E, and F) DSA on AP projection roadmap showing the maneuver of deployment for this case: navigation until M1 (C), distal initial deployment (D) and positioning on the planned landing zone (E and F). (G) Roadmap from DSA on lateral view showing the stent deployed over the curve. (H) Non-subtracted view showing the stent fully deployed. (I) DSA after the procedure showing contrast stagnation.
Patient 8 presenting with a cavernous compression syndrome and a partially thrombosed aneurysm. (A) 3DRA of the right internal carotid artery (ICA) showing the aneurysm and the distal and proximal landing zones of the carotid artery. Note the stenotic appearance due to compression of the artery by the aneurysm. (B) Preprocedure angiogram—working projection. (C) Non-subtracted single shot image showing the Pipeline Flex device deployed. (D) Right ICA angiogram/venous phase showing the contrast stagnation after device placement. (E) Right ICA angiogram/arterial phase after the procedure.
In cases where the distal landing zone is short, either close to an ICA curve or to the neck of the IA, we can deploy the device partially, recapture, and redeploy the device further distally before pulling it back to the planned landing position with a better placement and opening. With this technique, we observed that the device was fully opened immediately because the Teflon sleeves moved distally with the blood hemodynamics. Once the distal end of the stent was positioned in the landing zone, it was then gradually deployed using a balancing combination of pushing the stent wire and pulling back the microcatheter in order to keep the stent and the microcatheter in the isocenter of the vessel to facilitate spontaneous opening, similar to deployment of the previous Pipeline device.
In cases where the curve is tight or irregular, the system, including the stent wire and microcatheter, may be advanced forward together to improve its apposition to the vessel wall. When needed, resheathing was performed by advancing the microcatheter over the stent, respecting the ‘no return’ marker and avoiding too much forward pressure on the system. We realized that recapturing was improved when the angle between the partially deployed stent and the microcatheter is wide, whereas a steeper or more acute angle is an indication that there is an increased forward pressure on the system that would make recapturing more difficult. In these cases, pulling the microcatheter back before recapturing is advisable. Once the device is almost completely deployed, opened, and apposed to the wall, the final step is to push forward the stent wire and move the proximal marker of the stent distal to the microcatheter marker or just unsheath the microcatheter. There is no need to recapture the distal tip of the wire with the Teflon sleeves. However, it can be used to advance the microcatheter into the stent, or distal to it, if telescoping strategy is planned or if any internal manipulation is needed to improve apposition of the device.
Results
A total of 10 patients harboring 12 aneurysms were included from April 2014 to May 2014. Table 1 presents the patient demographics and aneurysm characteristics. The mean age of the patients was 57.7 years (range 27–75). A total of 12 stents were deployed, including 11 Pipeline Flex and one regular Pipeline device (short size not available in the Pipeline Flex sizes’ range). All targeted aneurysms were treated and all attempts at deployment were successful and no devices were discharged. The physicians’ evaluation of the procedure compared with their previous experience with the regular version of the Pipeline is described in table 2. Seven aneurysms were located at the paraclinoid region and three aneurysms were located at the cavernous portion. Three cases were part of a segmental arterial defect with multiple aneurysms or signs of vascular disease. Among 10 procedures, recapture and repositioning of the Pipeline Flex was performed five times with excellent performance, except in one case where we could not recapture it because of tight and multiple proximal curves on the ICA. However, in this case, stent wall apposition was not affected. We also found that the device opened more easily after recapture, and, therefore, we used it to improve opening or deployment in difficult anatomies.
Patient demographics and aneurysm characteristics
Technical nuances and expert opinion on each procedure comparing performance of Pipeline Flex and the previous Pipeline version, except resheathability maneuver, which can only be performed on the new version
One-layer device implantation was performed in all but one case. In two cases we performed prior coiling of the aneurysm before stent implantation because we judged that revealing symptoms of these IAs might have represented sentinel symptoms. No intraoperative complications were described. No side vessels were occluded at the end of any of the procedures. Contrast stagnation was seen in all cases and pronounced stasis was observed in five patients. All cone beam CT stenting imaging after the procedure demonstrated excellent apposition without irregularities or default of opening. In one case, a remodeling balloon was used to appose the proximal end of the stent that ended over a tight curve. Eight patients had an uneventful postoperative course, two patients presented postoperatively with transitory neurologic symptoms (monoparesthesia and blurred vision) that regressed spontaneously and completely. MRI/MR angiography showed stent patency and no new ischemic lesions or diffusion-weighted MRI positivity. Patients stayed for a maximum of 4 days in hospital and all were discharged home without complaints, with double antiaggregation for 3 months, aspirin for the following 9 months, and a further consultation scheduled for 6 months after follow-up imaging.
Discussion
We describe the safety and efficacy of the first experience with the newest generation of FDS, the Pipeline Flex device. The device was shown to be secure and changes made to the delivery system enabled successful deployment of the device as planned in all cases of this preliminary series. Independent evaluation of the device's apposition to the vessel wall was good or excellent in all cases. We used balloon remodeling to improve final wall apposition in only one case on the proximal part of a device that ended over the cavernous–petrous ICA curve. Resheatheability and the removal of the capture coil were perceived as major improvements by the operators. All devices that were intended to be implanted could be deployed and no device was discharged or had to be removed because of malfunction or wrong placement. Technical problems with FDS are under-reported, but in a recent series 13% of the previous version of Pipeline devices were discharged or removed.7 We are certain that the ability to recapture and reposition contributed to the success rate of implantation in our series.
We recaptured the device in 5 of 10 cases in order to reposition it more precisely, to improve apposition, or because it migrated proximally during deployment. We found that the device opened more easily (ie, earlier and more completely) after a first deployment attempt and recapture and we used this strategy subsequently to improve its opening and apposition in tight curves or complex loops. Sometimes, on tight curves or tortuous anatomic configurations it can be difficult to appose a flow diverting stent. This was particularly improved in the cases of this series with the Pipeline Flex, which could be better apposed without any additional maneuver after final placement. Improvements of the pusher wire increased pushability compared with the previous Pipeline version and with other FDS. We do not believe that this new device will require a long learning curve for physicians who have experience with the previous versions, but training for some adaptations of the deployment will be required. The new device opens immediately after being pushed outside the microcatheter, so no maneuver to open the stent needs to be performed. If the device is oversized at the distal landing zone or if the vessel is too small the device cannot fully open after first deployment, so we recommend recapturing the device forcing the Teflon sleeves distally, which then permits complete opening. The behavior of the device itself remains the same as that of the previous version.
FDS have been described as a major advance in the treatment of IAs.1 ,4 ,6 ,8 Complications with these devices include perioperative technical problems related to physicians’ experiences, difficult anatomy, or case complexity.9 ,10 Technically challenging maneuvers10–12 have been used to overcome some of these problems. Technical improvements for FDS deployment were deemed necessary to reduce technical complications in the management of IAs. Given our initial experience with this new device we believe that major improvements were made in the delivery mechanism without changing the successful and proven design of the FDS itself. The Pipeline device has had good clinical and long-term results in various previous studies,1 ,4 ,6 ,8 ,13 indicating that the stent design and material is appropriate for treatment of IAs.2 ,3 It can be assumed that the Pipeline Flex shares these properties since it kept the same stent design and material while only changing and improving the delivery mechanism.
We observed two transitory neurologic events up to 24 h after the procedure despite sufficient antiaggregation.14 Both patients improved rapidly without intervention and MRI showed no signs of ischemic events. The occurrence of thromboembolic events after neurovascular procedures is well known and diffusion-weighted MRI dots can appear in up to 50% of cases depending on the procedure time length, technique used, and patient’s age.14 ,15 These transitory ischemic events can be related to different steps of the procedure, including placement of the guiding catheter, microcatheter navigation, or stent deployment. We presume that by improving the deployment system, procedural times and related complications will decrease considerably.
We report here the first experience with the new Pipeline Flex device as well as immediate results and perioperative complications. Our results show that saccular aneurysms of the anterior circulation can be safely treated with the new Pipeline Flex device. Major improvements in the redesigned delivery system permitting resheathability and better support during deployment have been made with no change to the flow diverting stent itself. Although the delivery is considerably easier with this new device, certain technical nuances need to be kept in mind.
References
Footnotes
Contributors Conception: PVM, MK, TK, PV, K-oL; clinical data: AP, EM, HY, GE, VMP, PV; imaging review and follow up: TK, MK, K-oL; writing: VMP, MK, TK; review: VMP, PV, EM, GE, AP, K-oL, TK, MK, HY. All data from this study is included in this paper.
Competing interests None.
Ethics approval Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All data from this study is inside this paper.