Article Text
Abstract
Introduction Despite a decade of research into virtual stent deployment and the post-stenting aneurysmal hemodynamics, the hemodynamic factors which correlate with successful treatment remain inconclusive. We aimed to examine the differences in various post-treatment hemodynamic parameters between successfully and unsuccessfully treated cases, and to quantify the additional flow diversion achievable through stent compaction or insertion of a second stent.
Methods A systematic review and meta-analysis were performed on eligible studies published from 2000 to 2019. We first classified cases according to treatment success (aneurysm occlusion) and then calculated the pooled standardized mean differences (SMD) of each available parameter to examine their association with clinical outcomes. Any additional flow diversion arising from the two common strategies for improving the stent wire density was quantified by pooling the results of such studies.
Results We found that differences in the aneurysmal inflow rate (SMD −6.05, 95% CI −10.87 to −1.23, p=0.01) and energy loss (SMD −5.28, 95% CI −7.09 to −3.46, p<0.001) between the successfully and unsuccessfully treated groups were indicative of statistical significance, in contrast to wall shear stress (p=0.37), intra-aneurysmal average velocity (p=0.09), vortex core-line length (p=0.46), and shear rate (p=0.09). Compacting a single stent could achieve additional flow diversion comparable to that by dual-stent implantation.
Conclusions Inflow rate and energy loss have shown promise as identifiers to discriminate between successful and unsuccessful treatment, pending future research into their diagnostic performance to establish optimal cut-off values.
- flow diverter
- aneurysm
- blood flow
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
INTRODUCTION
Computational fluid dynamics has been used for over a decade to assess the hemodynamics of intracranial aneurysms after flow diversion treatment.1–6 This is because a favorable treatment outcome is largely associated with the efficacy of flow diversion produced by the implanted device; redirecting much of the aneurysmal inflow can ‘weaken’ the hemodynamic activity inside the sac, thereby promoting thrombosis and accelerating intracranial aneurysm occlusion.7
In view of this mechanism, a wide variety of hemodynamic parameters have been proposed to quantify the efficacy of flow-diverting (FD) stents: from those measuring reduction of the aneurysmal inflow strength (eg, aneurysmal inflow rate, intra-aneurysmal average velocity, mean aneurysm flow amplitude)8–11 to those measuring weakening of the vascular pressure or stress (eg, wall shear stress, pressure drop),2 12 13 and then to those characterizing changes in flow behavior within the aneurysm sac (eg, relative residence time, vortex core-line length).8 14 15 Furthermore, many research groups have also attempted to establish hemodynamic identifiers of a successful treatment,4 9 12 13 16–19 although no consensus has yet been reached.
Owing to the development of virtual stenting techniques,6 20 21 computational fluid dynamics has also been adopted to investigate the changes in aneurysm hemodynamics following various strategies or maneuvers exercised intra-operatively to enhance the flow diversion, in particular stent compaction and multiple stent deployment. Despite a number of simulation studies having confirmed the effectiveness of these approaches in enhancing the flow diversion,3 18 19 22 it remains inconclusive as to how much further an additional stent or a stent compaction technique can decrease the aneurysmal inflow.
In the present work we performed a pooled analysis of eligible studies on the aneurysmal hemodynamics after virtual stent treatment, seeking to (1) identify hemodynamic parameters that may be most suggestive of a favorable treatment outcome and (2) quantify the likely additional flow diversion achievable through a stent compaction technique or insertion of an additional stent.
METHODS
Study selection
We conducted the systematic review and meta-analysis in accordance with the PRISMA guidelines. A search for the keyword terms “computational fluid dynamics”, “haemodynamics” or “hemodynamics”, in combination with “aneurysm(s)”, “flow-diverter(s)” or “flow-diverting stent(s)” was performed using PubMed, Medline, and Scopus for articles published in the period from 1 January 2000 to 31 December 2019. From the identified studies, review articles, articles that were written in languages other than English, technical notes, and letters were immediately excluded.
For the remaining articles, three authors (MZ, ST, and MO) independently examined the full text before reaching an agreement on their eligibility to be included in our quantitative analysis according to the following criteria: (1) human aneurysm subjects were included; (2) paired pre- and post-stenting hemodynamic parameters were quantified; and (3) clinical outcomes following a treatment were disclosed (if it was to be analysed for the correlation between hemodynamic changes and clinical outcomes). When serial studies existed describing the results of an overlapping set of subjects, only the most recent one was retained (see figure 1 for the literature search strategy used to screen papers for our quantitative analyses).
Strategy used for literature search and the number of studies included in each analysis.
Data extraction and normalization
For quantitative analysis we extracted data including (1) name of the first author, (2) year of publication, (3) aim and design of the study, (4) number, type, size, and location of the aneurysms, (5) type of the stents and strategy used for virtual deployments, and (6) all available pre- and post-treatment hemodynamic parameters. These parameters included the aneurysmal inflow rate (IR), intra-aneurysmal average velocity (AAV) and maximal velocity (MAV), average shear strain rate (SR) of blood flow within the aneurysms, energy loss (EL), pressure drop (PD), aneurysmal wall shear stress (WSS) and vortex core-line length (VCL), the relative residence time (RRT) and turnover time (TUT), and transition time (TRT). Two authors (HA and YK) independently performed data extraction, the results of which were consistent.
As the extracted data were reported in various units and formats across different studies, a data normalization procedure was carried out to ensure validity of between-study comparisons of the hemodynamic outcomes of the treatments. Paired hemodynamic parameters in physical units before ( ) and after (
) and after ( ) treatment respectively in the forms of
) treatment respectively in the forms of
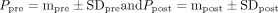 (1)
(1)
were converted into a normalized reduction  in relation to the untreated condition:
in relation to the untreated condition:
 (2)
(2)
where  and
and 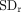 denote the mean and SD of the normalized reduction, which were respectively calculated as23
denote the mean and SD of the normalized reduction, which were respectively calculated as23
 (3)
(3)
and
 (4)
(4)
Statistical analysis
Statistical analyses were performed using MedCalc version 19.1 (Ostend, Belgium), with GNU Octave 4.4.1 (John W Eaton et al, USA) being a supplementary tool. For systematic comparisons between the successfully and unsuccessfully treated groups, we first performed a Shapiro–Wilk test to check for normality of the continuous data, and then an independent Student t-test for normally distributed data or a Mann–Whitney U test for non-normally distributed data. For comparison of hemodynamic changes between various treatment configurations (ie, treatments with single or with dual FD stents, and treatments with or without a stent compaction technique applied), a paired-sample two-sided t-test was used. Throughout the comparisons p<0.05 was considered suggestive of statistical significance, while p<0.005 was deemed to confirm statistical significance.
A meta-analysis was performed to explore the standardized mean difference (SMD) of six hemodynamic parameters (IR, AAV, EL, VCL, WSS, and SR) between the successfully and unsuccessfully treated groups. Both fixed-effects and random-effects models were used in each analysis, with a Cochran Q test employed to examine the between-study heterogeneity. If a test had suggested significant heterogeneity between studies, results corresponding to a random-effects model were adopted, otherwise results corresponding to a fixed-effects model were adopted. We used funnel plots and Egger’s test to examine the publication bias, with p<0.05 being an indication of statistical significance.
Results
Our database search yielded 259 studies after duplicates were removed, of which 38 were deemed eligible for further quantitative analysis.
For systematic analysis of the correlation between hemodynamic and clinical outcomes, 12 studies (involving a total of 153 aneurysms) with clinical outcomes disclosed were included. Of the 12 studies, five with aneurysm sample size greater than five were later included in the meta-analysis (see table 1 for a summary of the studies). We observed no substantial asymmetry in the funnel plots of studies that reported IR or AAV, and Egger’s tests did not suggest the existence of publication bias (p=0.78 and p=0.89 for IR and AAV, respectively).
Study information and normalized reductions relative to the untreated condition of hemodynamic parameters summarized from studies included in the meta-analysis and systematic review for differences between successfully and unsuccessfully treated cases
Of the 38 studies, 11 met the inclusion criteria for quantifying the improvements of flow diversion achieved by two respective wire density enhancement techniques—stent compaction (7/11) and dual-stent implantation (5/11) (see online supplemental material 1 for a list of those studies, online supplemental table 1 for a summary, and online supplemental table 2 for details of the normalized hemodynamic parameters extracted from those studies).
Supplemental material
Supplemental material
Hemodynamic differences between successfully and unsuccessfully treated cases
Quantitative comparison
Our quantitative analyses of 12 studies suggest that the differences in IR (p=0.0063) and EL (p=0.0237) between the successfully and unsuccessfully treated aneurysms were suggestive of statistical significance. However, no statistically significant differences were found for AAV (p=0.2881) or WSS (p=0.8280) (see online supplemental figure 1 for the detailed quantifications and online supplemental material 2 for an additional logistic regression analysis).
Supplemental material
Meta-analysis
Consistent with the findings of the quantitative comparison, our meta-analysis of five studies (including a total of 138 aneurysms) shows the differences in normalized IR (SMD −6.05, 95% CI −10.865 to −1.225, p=0.01) and EL (SMD −5.28, 95% CI −7.092 to −3.459, p<0.001) are indicative of statistical significance, in contrast to AAV (SMD −3.22 95% CI −6.985 to 0.537, p=0.09) and WSS (SMD 0.34, 95% CI −0.411 to 1.083, p=0.37) (figure 2 and table 1).
Meta-analysis of standardized mean differences (SMD) of six different hemodynamic parameters (I–VI) and clinical treatment outcomes using both the fixed-effects and the random-effects models. The forest plots show SMDs of each of these parameters obtained from both the fixed-effects (upper rhombus) and the random-effects (lower rhombus) models.
Improvement of flow diversion obtained by increased stent wire density
Table 2 summarizes the improvements in intra-aneurysmal flow reduction obtained by enhancement of the stent wire density across the aneurysm orifice. Our systematic review suggests that overlapping two stents and compacting a single stent can each markedly improve the flow diversion efficacy, further reducing the IR by 14.4±5.6% (95% CI 9.3% to 19.6%, p=0.0005) and 16.0±11.3% (95% CI 6.7% to 25.4%, p=0.0051), respectively, and the AAV by 16.8±8.7% (95% CI 10.1% to 23.5%, p=0.0004) and 23.3±14.4% (95% CI 8.3% to 38.4%, p=0.0105) compared with the standard deployment of a single stent. However, the increase of normalized TUT induced by stent compaction was not suggestive of statistical significance (p=0.0791), in contrast to that induced by overlapping stents (p=0.0247) (see online supplemental figures 2 and 3 for statistics on the hemodynamic changes attributable to increasing the stent wire density across the aneurysm orifice).
Change of hemodynamic parameters (relative to the untreated condition) after deployment of an additional stent or when a single stent was deployed with a compaction technique applied compared with standard deployment of a single stent
Discussion
IR and EL reduction might be suggestive of a successful treatment
Our quantitative comparison suggests that only differences in the normalized IR and EL reductions between the successfully and unsuccessfully treated groups were suggestive of statistical significance, and this finding agrees with our meta-analysis.
IR is a so-called 'bulk' hemodynamic parameter measuring the entire quantity of blood flow entering the aneurysm. Strength and angle of the aneurysm inflow have direct impact on the complexity and stability of flow within the aneurysm sac, which are believed to be closely associated with aneurysm occlusion or rupture.7 13 As a parameter originally proposed to quantify the hemodynamic effects of different anastomosis modes in cardiovascular surgery, EL measures differences in the dynamic pressure and kinetic energy between the aneurysmal inflow and outflow. Being able to immediately quantify the weakening of intra-aneurysmal flow activity, IR and EL have both been proposed as identifiers of a fast aneurysm occlusion; however, differing conclusions were previously reported regarding their association with clinical outcomes.
Paliwal et al 16 reported that, superior to bulk hemodynamic parameters (ie, IR (p=0.10) and AAV (p=0.34)), localised parameters (ie, EL and VCL (both p<0.00)) were more sensitive in capturing the complex intra-aneurysmal flow behaviors, which are likely to manifest greater disruption in successfully treated intracranial aneurysms (table 1). Interestingly, in another study8 that involved more aneurysm samples (n=84), reductions of the IR, AAV, and SR were all found to be statistically different (all p<0.00), in contrast to TUT (p=0.47), a parameter measuring the average period of time during which fluid particles remain within the aneurysm sac. Chong et al 9 and Mut et al 13 also reported the difference in IR to be statistically significant (both p<0.00), although the former found that the difference in EL between the successful and unsuccessful cases was about three times that of IR.
Animal experiments showed a similar tendency when hemodynamic parameters were used to predict complete aneurysm occlusion. A study of 36 elastase-induced aneurysms in rabbits reported statistically significant differences in IR (p<0.03) and kinetic energy (p<0.05) between fast and slow aneurysm occlusion, in contrast to that of AAV (p=0.06), regardless of aneurysm size.14 Moreover, their follow-up research into the intra-aneurysmal hemodynamics showed that AAV and SR in the patent regions were about 2.8 times larger than those in the occluded regions (p<0.00),7 suggesting the usefulness of AAV and SR in predicting localized thrombosis formation.
In accordance with our review of studies on human subjects, together with the findings from animal experiments, IR and EL have shown promise as discriminators between favorable and unfavorable clinical outcomes prior to a real flow diversion treatment, pending further investigation into their diagnostic performance to identify the optimal cut-off thresholds.
Additional flow diversion achievable through enhancement of stent wire density
In addition to deployment of an additional stent, a 'push-and-pull' technique to be used during the stent-releasing process has been promoted to enhance the local wire density. Shapiro et al 24 first demonstrated in benchtop experiments that increased wire density can be attained through longitudinal compaction of a single stent. Damiano et al 22 later reported in a hemodynamic study that compacting a single FD stent may outperform deployment of two stents in producing additional reductions of the IR, AAV, and mean WSS.
The results of our systematic review confirmed that stent compaction and dual-stent deployment could both improve the flow diversion efficacy in terms of further reductions of IR (p<0.006) and AAV (p<0.02), which are both indicative of statistical significance. However, stent compaction did not demonstrate statistically significant further improvement of the TUT (p=0.08), in contrast to the deployment of an additional stent (p=0.02). This suggests that bulk hemodynamic parameters may be more prominently affected by a general increase in the stent wire density whereas localized parameters may be more sensitive to how the specific wire configuration was altered across an aneurysm orifice.
Furthermore, compacting a stent may be inappropriate when a patient has a highly curved recipient artery with an aneurysm located at the apex of the curvature. Zhang et al 18 pointed out that, in this circumstance, the stent wires might be pushed into the sac of an aneurysm due to the restoring force acting inside the wires, counter-productively resulting in a lower metal coverage ratio across the aneurysm orifice, causing treatment failure.
It should also be noted that enhancement of wire density through implantation of an additional stent may further impair patency of the side-branch arteries jailed by the implanted device.25 Moreover, stent compaction or dual-stent deployment may increase the occurrence of post-treatment in-stent thrombosis, even when adequate dual antiplatelet therapy is administered.26 This is still beyond the capability of numerical simulation to accurately predict. Therefore, although flow diversion can be improved through enhancement of stent wire density, whether these techniques can be applied must still take into consideration the patient-specific vascular conditions.
Uncertainties in virtual stent deployment and hemodynamic simulation
The simulation strategies reported in our surveyed studies were based on various combinations of modeling techniques, with few of them being able to be rigorously validated against clinical practice.
The primary factor limiting the fidelity of virtual stent deployment is that the wire configuration of stents deployed in vivo depends substantially on the skill, experience, and predilection of the treating clinicians, especially when a patient exhibits variation or tortuosity in vascular geometry, or for aneurysms with uncommon morphology in difficult-to-approach locations. Even under ideal vascular conditions, the landing position of the stent, the unsheathing process, and the pathway along which a stent is released can be quite different from the scenario modeled by virtual stent deployment.
In addition, only a limited number of studies have evaluated the local microscopic properties (eg, porosity and pore density) of stent wires at the aneurysm orifice. Such data would provide a better understanding of the efficacy of the stent in terms of aneurysmal IR and EL reduction in relation to specific vascular geometries with variations in diameter and curvature of the parent artery.
For analysis of the intra-aneurysmal hemodynamics, the key factors that may be detrimental to simulation accuracy are the adoption of generic vascular boundary conditions (eg, for flow rate and pressure) or the prescription of unphysiological velocity or pressure profiles at vascular openings. In most hemodynamic analyses, generalized inlet flow rates and outlet pressures—rather than patient-specific hemodynamic conditions—were assumed. This may cause substantial under- or over-estimation of the actual flow diversion produced by a stent, as the aneurysmal inflow was reported to be linearly proportional to the strength of flow in the parent artery.27 28 A recent study by Najafi et al 29 suggested that adoption of a cycle-average internal carotid artery flow rate derived from patient-specific phase-contrast MRI may help reduce error.
Furthermore, various assumptions were applied in hemodynamic simulation to simplify the description of blood flow (eg, assumption of a Newtonian fluid with constant viscosity and density). Saqr et al 30 recently concluded that the assumption of a Newtonian fluid may be invalid, especially when parameters quantifying the flow close to the vascular wall (eg, WSS) are to be assessed. Other simplifications include assuming the vascular wall to be rigid (ie, the wall would not deform over a cardiac cycle or even after implantation of a FD stent). However, Bouillot et al 31 have recently shown that deformation of the parent artery can be substantial after stent implantation, and Tupin et al 32 reported that the intra-aneurysmal flow pattern may be markedly affected by the compliance of the wall.
All these uncertainties and assumptions may lead to discrepancies between the in vivo aneurysmal hemodynamics and the simulated ones, yet the current imaging techniques lack the capability to capture the subtle in vivo flow patterns within an aneurysm, which could have been used as gold standard to regulate hemodynamic simulation. As a future direction, comprehensive validation and standardization of the protocols for virtual stent deployment and hemodynamic simulation would be useful, after which cogent results of virtual treatment may be used by clinicians in assessing the outcomes prior to real flow diversion treatment.
Limitations
Pursuant to the source studies, the present review has several limitations. The major one is that, although we have surveyed almost all studies ever published that reported post-treatment hemodynamics and aneurysm occlusion status, only five studies met our inclusion criteria for meta-analysis and even then most of the included studies had relatively small sample sizes. The reason is that performing virtual stent deployment and the subsequent hemodynamic analysis demands cutting-edge simulation skills and has a high resource intensity in terms of both manual interaction and computation time. Whether the conclusions drawn from our meta-analysis still hold for a larger group of aneurysm samples deserves further investigation.
The second inherent limitation is that the timepoints at which aneurysm occlusion status was observed varied from 3 to 12 months across the studies we included. Different research groups determined distinct endpoints following their local protocols for postoperative follow-ups. Although the criterion for classifying a treatment as successful was consistently specified as complete occlusion, lack of consistency among the specified observation times may potentially lead to misclassification of some treatment outcomes according to any single standard, thereby clouding exploration of the correlation between the hemodynamic and clinical outcomes.
Another limitation is that we have included two studies from the same group in our meta-analysis, which may potentially lead to duplicates of the included data. However, these two studies reported and focused on different hemodynamic parameters, and had different timepoints at which the status of aneurysm occlusion was assessed. Moreover, we failed to identify any overlapping cases through close examination of the aneurysm geometries.
Conclusion
Clinical outcomes following flow diversion treatment were found to be associated with the simulated aneurysm hemodynamics.
Differences in the post-treatment IR reduction (SMD −6.05, 95% CI −10.87 to −1.23, p=0.01) and EL reduction (SMD −5.28, 95% CI −7.09 to −3.46, p<0.001) between successfully and unsuccessfully treated patients were indicative of statistical significance, in contrast to the AAV (p=0.09), mean WSS (p=0.37), SR (p=0.09), and VCL (p=0.46). Deployment of stents with a compaction technique may yield additional enhancement of flow diversion comparable to that achieved through deployment of an additional stent.
Aneurysmal IR and EL have shown promise in discriminating between favorable and unfavorable patient outcomes following flow diversion treatments, pending future large studies into the diagnostic performance to establish the optimal cut-off thresholds.
Acknowledgments
We sincerely appreciate the support received from our CFD-BIO study team, who gathered together in a special workshop held in Japan (July 2019), having had fruitful discussions on topics related to the current study and contributed tremendously to the processing of data reported in this article. They are F Ishida (Mie Central Medical Center); T Sano (Ise Red Cross Hospital); Y Umeda (Mie Prefectural General Medical Center); M Beppu (Hyogo Medical College); M Nakamura, T Yamada, and Y Yamamoto (Nagoya Institute of Technology); S Nemoto (Tokyo Medical and Dental University); S Shinozaki and N Okada (Waseda University); H Ohno, T Ishii, T Okudaira, Y Yamanaka, and F D Wakabayashi (Tokyo University of Science); F Pan (Tohoku University); Y Shimogonya (Nihon University); S Fukuda (National Hospital Organization Kyoto Medical Center); K Kanou (Asahi University); and S Kuwahara (Kinki Central Hospital).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @SimonTupin
Contributors Guarantor of integrity of entire study: MO. Study concepts/study design: TY, MS, MO, SF, YO, KS and KT. Data acquisition: all authors. Data extraction and analysis: MZ, ST, MO, YK, and HA. Manuscript drafting: MZ. Manuscript revision for important intellectual content: all authors. Approval of final version of submitted manuscript: all authors. Agrees to ensure any questions related to the work are appropriately resolved: all authors. Statistical analysis: MZ, ST, MO, YK and HA. Manuscript editing: all authors.
Funding The authors acknowledge the support received from the Grant-in-Aid for Scientific Research (B), Japan Ministry of Education, Science, Sports and Culture (JP20H04557, Makoto Ohta).
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.




