Article Text
Abstract
Background Thromboembolic complications (TECs) are frequent during the endovascular treatment of unruptured aneurysms. To prevent TECs, dual antiplatelet therapy using aspirin and clopidogrel is recommended for the perioperative period. In patients with a poor response, clopidogrel is a risk factor for TECs. To prevent TECs, our study assessed the stratified use of prasugrel.
Methods Patients who underwent endovascular therapy for unruptured cerebral aneurysms from April 2017 to August 2019 were enrolled in this clinical study and given premedication with aspirin and clopidogrel for 2 weeks prior to the procedure. P2Y12 reaction units (PRU) were measured using the VerifyNow assay on the day before the procedure (tailored group). In subgroups with PRU <240, the clopidogrel dose was maintained (CPG subgroup). In subgroups with PRU ≥240, clopidogrel was changed to prasugrel (PSG subgroup). We compared the occurrence of TECs with retrospective consecutive cases from January 2015 to March 2017 without PRU assessments (non-tailored group). The frequency of TECs within 30 days was assessed as the primary endpoint.
Results The tailored and non-tailored groups comprised 167 and 50 patients, respectively. TECs occurred in 11 (6.6%) and 8 (16%) patients in the tailored and non-tailored groups (P=0.048), respectively. The HR for TECs was significantly reduced in the tailored group (HR 0.3, 95% CI 0.11 to 0.81); P=0.017) compared with the non-tailored group.
Conclusion The results suggest that tailored dual antiplatelet therapy medication with PRU significantly reduces the frequency of TECs without increasing hemorrhagic complications.
- aneurysm
- catheter
- embolic
- hemorrhage
- intervention
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
Thromboembolic complications (TECs) are the most common complications of endovascular therapy for an unruptured intracranial aneurysm (UIA). These often result in severe complications and stent thrombosis, among others. The incidence of postoperative TEC after endovascular therapy for UIA has been estimated to be 7.3–15.8%.1–3 To prevent TECs, dual antiplatelet therapy (DAPT) is recommended for the perioperative period.4 Aspirin (Bayaspirin, Bayer, Leverkusen, Germany) and clopidogrel bisulfate (Plavix, Sanofi, Paris, France) are commonly prescribed as the perioperative DAPT. However, in patients with a poor response to clopidogrel, taking clopidogrel for endovascular therapy is a thromboembolic risk factor.5 Approximately 18–23%, up to 70%, of Asians are resistant to clopidogrel, while only 3% of Caucasians are resistant to it.6 7 This is due to the higher presence of single nucleotide polymorphisms in cytochrome P450 2C19 among Asians.8 Prasugrel (Effient, Daiichi Sankyo, Tokyo, Japan), which is a third-generation thienopyridine that does not have as varied effects across individuals as clopidogrel, is widely used in the practice of coronary intervention.9 10 Clopidogrel is a prodrug belonging to the thienopyridine class of antiplatelet medications. Once activated by the cytochrome P450 system, the active metabolite binds to the P2Y12 receptor, thus inhibiting ADP receptor-mediated activation. Prasugrel also acts by inhibiting ADP receptor-mediated activation of platelets. It requires only a one-step activation, enables more effective platelet inhibition, and does not incur heterogeneous responses across individuals, unlike clopidogrel.9 10 The purpose of this study was to assess the stratified use of prasugrel dependent on platelet activity in order to prevent TECs in endovascular therapy of UIAs.
Methods
Patient population, study design, and definition of each group
This study was a single-center prospective study for tailored administration of thienopyridine dependent on platelet reactivity (tailored group) based on the following inclusion/exclusion criteria collected between April 2017 and August 2019. The tailored group was further divided into two subgroups: a ‘CPG subgroup’ in which the patients were pretreated with clopidogrel, and a ‘PSG subgroup’, in which the patients were switched to prasugrel as described below. A comparative study of prospective data (tailored group) was conducted with a retrospective cohort (non-tailored group). The historical data of 50 consecutive patients between January 2015 and March 2017 with the same criteria were included (figure 1).
Study flow chart. ASA, aspirin; CPG, clopidogrel; PSG, prasugrel.
Inclusion criteria
Consecutive patients with any of the following characteristics were included:
patients with scheduled neurointervention for UIA aged 20–80 years who were pretreated with DAPT as per the regimen described below;
patients who underwent diffusion-weighted imaging (DWI) on MRI within 5 days following the procedure.
Exclusion criteria
We excluded patients with any of the following characteristics:
taking any anticoagulation or antithrombotic agent (eg, warfarin, direct oral anticoagulation or other antiplatelet therapy (eg, cilostazol));
any platelet count abnormality (<50 000/μL, normal value >158 000/μL);
any hemorrhagic disease (eg, gastrointestinal hemorrhage);
allergy to aspirin, clopidogrel, or prasugrel;
scheduled parent artery occlusion; and
any other conditions per researchers’ judgment.
Periprocedural antiplatelet medication and measurement of platelet activity
The VerifyNow assay (Accumetrics, San Diego, California, USA) was used to measure platelet reactivity. Reduced effectiveness of P2Y12 receptor antagonist therapy is represented by a high P2Y12 reaction unit (PRU) value. It tends to be linked to a higher risk of TECs, according to the literature, for PRU values >240.10 For 2 weeks before the procedure, patients received 100 mg/day aspirin (Bayaspirin, Bayar, Leverkusen, Germany) and 75 mg/day clopidogrel (Plavix, Sanofi, Paris, France) once a day. The day before the procedure the patients were admitted and their PRU and aspirin reaction unit (ARU) values were measured. If the PRU value was <240, the clopidogrel dose was maintained (CPG subgroup). If the PRU value was ≥240, the P2Y12 antagonist was changed from clopidogrel to prasugrel (PSG subgroup). A loading prasugrel dose of 20 mg/day was administered, followed by 3.75 mg/day. On the day of the procedure, patients in the PSG subgroup had their PRU value measured again on the morning before the procedure, and all patients underwent the endovascular procedure. When the PRU value was <100 on the day of the procedure in the PSG subgroup, we reduced the dose of PSG by half (1.875 mg). In the non-tailored group, the antiplatelet schedule was the same as in the CPG subgroup—that is, aspirin and clopidogrel were administered and the platelet reactivity was not measured. A high PRU cut-off value of 240 was selected, according to the study by Delgado et al.11 A low PRU cut-off value of 100 was also selected, according to the studies of Sambu et al 12 and Stone et al 10 (figure 1).
After the procedure, patients underwent antiplatelet therapy as directed. Patients who underwent a coil embolization procedure alone took aspirin for 3 months and clopidogrel or prasugrel for 3 days. Patients who underwent stent-assisted coil embolization took aspirin for 1 year and the other antiplatelet medications for 3 months. Patients who had a flow diverter placement took aspirin for at least 1 year and the other antiplatelet medications for 6 months.
Endovascular treatment procedure
The procedure was performed under general or local anesthesia by a team of neurointerventionists through transfemoral access. Systemic heparinization (Heparin Sodium, Mochida Pharmaceutical, Tokyo, Japan) was administered intravenously after insertion of the sheath introducer, with a targeted activated clotting time of >250 s, or twice for the control count (the normal value was 100–150 s at our institution). The treatment options were coil embolization, balloon-assisted coil embolization, stent-assisted coil embolization, and flow diverter placement, depending on the patient. A final biplanar angiography was performed to document the patency of the intracranial vasculature. Hemostasis was achieved with an Angio-Seal device (St Jude Medical, Minnetonka, Minnesota, USA). Heparinization was terminated at the end of the procedure without reversal.
MRI
The DWI was performed in all patients in both the tailored and non-tailored groups, as the post-treatment MRI is performed even outside the study as routine examination in our institute. The DWI positive findings after cerebral endovascular therapy without neurological findings were defined as clinically silent ischemic lesions (CSILs).13 14 A total of 5–60% of patients had CSILs after the procedure.13 According to previously published studies, individuals with findings ≤10 mm in maximal diameter on axial DWI without any neurological deficits were eligible for CSILs.14 With regard to this study, the MRI which included DWI, fluid-attenuated inversion recovery, T2-weighted imaging, and magnetic resonance angiography was performed within 5 days after the endovascular procedure. The high intensity area (HIA) on DWI was assessed by a radiologist not related to the procedure who made a blind diagnosis. We created our own DWI grading scale based on the previously published literature: Grade A, no HIAs; Grade B, small HIAs (≤5 spots and each ≤10 mm); Grade C, some small HIAs (>5 spots and each ≤10 mm); Grade D, large HIAs (at least one spot >10 mm); and Grade E, not examined because of aneurysmal rupture or coma.
Endpoints and definitions
Primary endpoint
The primary endpoint was symptomatic intracranial TECs, defined as a DWI-positive image with neurological findings of a transient ischemic attack or cerebral infarction developing within 30 days after the procedure.15
Secondary endpoints
The secondary endpoints were the following:
Complications: we used the symptomatic hemorrhagic complications and all symptomatic complications in the 30 days after the procedure, without counting intraoperative rupture associated with coil treatment because it is less relevant to complications associated with antiplatelet treatment. The International Society for Thrombosis and Haemostasis (ISTH) major bleeding criterion was used to define ‘symptomatic hemorrhage’.16
DWI grading: we used the frequency and severity of TECs assessed by DWI of MRI within 5 days after the procedure.
PRU cut-off in terms of TECs: the cut-off PRU values in terms of TECs evaluated by a receiver operating characteristic (ROC) curve in the tailored group.
Statistical methods
Continuous variables were presented as means and SDs and compared with a t-test and Fisher’s exact test, and PRU values were compared with a paired t-test. We used the Cox proportional hazards regression model adjusted for covariates including age, sex, location, and device. Hazard ratios and 95% CIs were used. We also calculated the area under the ROC curve (AUC) and the threshold of the PRU value. AUC and the threshold were internally validated using the bootstrap method with 10 000 resamples. The bootstrap bias-corrected AUC (bootstrap AUC-ROC) was reported as the measure of the predictive performance of the model. The average of the thresholds obtained from each of the 10 000 resamples was considered the most clinically useful cut-off point. A two-sided 5% significance level was considered statistically significant. All statistical analyses were performed using R (version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, 2019).
Results
Background characteristics
In this prospective cohort study, 167 patients were available for analysis in the tailored group while four patients withdrew their consent. Among these, 140 patients (84%) were classified in the CPG subgroup and 27 patients (16%) were classified in the PSG subgroup according to the evaluation of PRU values on the day before treatment (figure 1). The tailored group was compared with the non-tailored group, comprising the retrospective cohort of 50 patients as described above. In the total of 217 patients there were 219 aneurysms. Background characteristics are shown in online supplementary table S1. The mean±SD age was 60.9±12.3 years (61.6±12.5 years in the tailored group and 58.4±11.4 years in the non-tailored group). There were 71 men (33%) and 146 women (67%). A total of 164 (75%) aneurysms were in the anterior circulation (internal carotid artery, anterior cerebral artery, anterior communicating artery, and middle cerebral artery) and 55 (25%) were in the posterior circulation (basilar artery and vertebral artery). All patients were treated using devices which were deliberately selected before surgery; coil embolization was performed in 66 (30%), stent-assisted treatment in 124 (57%), and a flow-diverter system was used in 24 (11%) patients. There was no statistically significant difference in background characteristics between the tailored and non-tailored groups, except for the endovascular procedure and the device used (P=0.46), which were mainly due to the introduction of flow diverter treatment in 2017.
Supplemental material
Response to various antiplatelet medications
The mean±SD PRU value was 159.5±49.8 in the CPG subgroup and 270.7±21.3 in the PSG subgroup on the day before the procedure. On the day of the procedure, the mean PRU value in the PSG subgroup was 95.1±54.8, which was significantly lower than that on the day before the procedure due to conversion of CPG to PSG (P<0.001) (figure 2). The preoperative mean±SD ARU value was 459.5±75.5 in the CPG subgroup and 438±117.4 in the PSG subgroup (P=0.143).
Mean preoperative P2Y12 reaction units (PRUs) measured with VerifyNow in the tailored group. Preoperative PRU values were measured on the day before the procedure. If the PRU value was <240, the clopidogrel dose was maintained (CPG subgroup). In patients with a PRU value ≥240, the P2Y12 antagonist was changed from clopidogrel to prasugrel (PSG subgroup). For the PSG subgroup, the PRU value was measured again on the day of the procedure (PSG subgroup, post).
Primary endpoint
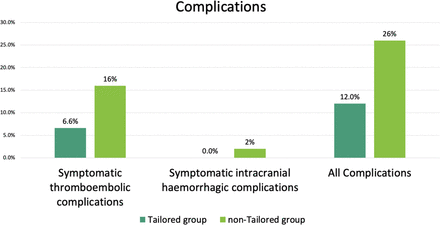
For the primary outcome, symptomatic TECs were observed in 11 patients (6.6%; 10 (7.1%) in the CPG subgroup, 1 (3.7%) in the PSG subgroup), and 8 (16%) in the non-tailored group (figure 3). Figure 4 shows the symptomatic TECs within 30 days using the Kaplan–Meier method. The adjusted HR for the symptomatic TECs was significantly reduced in the tailored group (HR 0.3, 95% CI 0.11 to 0.81; P=0.017) compared with the non-tailored group.
Incidence of symptomatic complications in patients within 30 days of the procedure.
Kaplan–Meier analysis of symptomatic thromboembolic complications.
Secondary endpoints
Complications
Symptomatic intracranial hemorrhagic complications affected no patients in the tailored group and one (2%) in the non-tailored group.16 Other symptomatic hemorrhagic complications that did not meet other major ISTH criteria were as follows: one retroperitoneal hematoma, five inguinal subcutaneous hematomas, one hematuria. They were included in all symptomatic complications.
All symptomatic complications within 30 days occurred in 20 patients (12%) in the tailored group (18 (12.9%) in the CPG subgroup and 2 (7.4%) in the PSG subgroup) and 13 (26%) in the non-tailored group. The HR for all symptomatic complications was 0.39 (95% CI 0.19 to 0.83; P=0.0141) compared with the non-tailored group.
DWI grading
DWI was performed in all patients, except those with a poor general condition due to intraoperative rupture (Grade E; one patient in the tailored group and one in the non-tailored group). With regard to the frequency and degree of perioperative TECs by MRI-DWI, Grades B and C were more common in the tailored group (Grade B: 60 patients (36%); Grade C: 55 patients (33%)) than in the non-tailored group (Grade B: 11 patients (22%); Grade C: 23 patients (46%)) (see online supplementary table S2, online supplementary figure S1). We assessed the associations of DWI grade using proportional odds logistic regression models. The adjusted OR for the tailored group compared with the non-tailored group was 0.48 (95% CI 0.26 to 0.88; P=0.017) after adjusting for age and sex (see online supplementary figure S2).
PRU cut-off in terms of TECs
An ROC curve was generated and the AUC (and its 95% CI) was calculated to determine the lowest TECs. An ROC curve analysis assessing TECs confirmed a PRU value of 175.5 (AUC 0.59, sensitivity 0.44, and specificity 0.59 (95% CI for AUC 0.44 to 0.59); sensitivity, 0.73; specificity, 0.54) (see online supplementary figure S3). The bootstrap AUC-ROC was 0.61 and the average of the thresholds obtained from each of the 10 000 resamples was 180.3.
Discussion
Although thienopyridine is a prodrug that requires conversion to active metabolite before irreversibly binding to the platelet P2Y12 receptor, prasugrel, which is a third-generation thienopyridine, has a simpler metabolic pathway and more prompt antiplatelet effect than clopidogrel.7 8 In our study, tailored antiplatelet medications using PRU as an index reduced the TECs compared with standard DAPT using aspirin and clopidogrel in a non-tailored cohort, without increasing hemorrhagic complications. This suggests that switching from clopidogrel to prasugrel while measuring PRU values significantly improved unstable platelet inhibition in patients who were clopidogrel poor responders.
Prasugrel dosage
Prasugrel is a third-generation thienopyridine and does not have as varied effects across individuals as clopidogrel, which has been widely used in the practice of coronary intervention since 2016. Prasugrel is commonly used as a global treatment in coronary intervention. The TRITON-TIMI 38 study compared prasugrel (60 mg loading dose and 10 mg daily maintenance dose) with clopidogrel (300 mg loading dose and 75 mg daily maintenance dose) in patients with acute coronary syndrome scheduled for percutaneous coronary intervention.9 In this study, the rate of occurrence of cardiovascular events was significantly reduced in the prasugrel group while major bleeding, including fatal bleeding, occurred more frequently. Reduced doses of prasugrel (20 mg loading dose and 3.75 mg daily maintenance dose) were administered in a study of coronary intervention involving Japanese patients who generally tend to be older and have a lower body weight, whereas a 60 mg loading dose and 10 mg daily maintenance dose are used globally.17 In this study, a reduced dose of prasugrel resulted in a lower incidence of cardiovascular events, although the difference was not statistically significant because of the small sample size. The incidence of bleeding was similar in both groups. Based on these studies, there has been a recent update of the guideline for coronary intervention recommending low-dose prasugrel for Japanese patients.18
With regard to the role of prasugrel as a neurointerventional strategy, two pioneering studies in the USA were reported in 2013.19 20 Specifically, Akbari et al reported that a significantly higher risk of hemorrhagic complications was observed with prasugrel (loading dose 60 mg with maintenance dose 10 mg/day) among the poor responders to clopidogrel (percent inhibition <40%). Nevertheless, there were many differences in TECs between the two thienopyridines in 51 patients with 55 procedures for various endovascular treatments, such as UIA, dural arteriovenous fistulas, and intracranial arterial stenosis (IAS).19 In contrast, there were no significant differences in TECs and hemorrhagic complications between clopidogrel and prasugrel for poor responders to clopidogrel (percent inhibition <20%, loading dose 40 mg with maintenance dose 5–10 mg/day) when assessed in 16 patients with UIAs and IAS.20 An additional report from France similarly indicated no differences in TECs and hemorrhagic complications between clopidogrel and prasugrel (100 patients in each group) using the same doses as those detailed by Akbari et al for stent-assisted coil embolization for UIA.21
Meanwhile, a series of studies with low-dose prasugrel were conducted by a Korean group for UIA.15 22–24 In these studies, loading doses of 20–30 mg were followed by maintenance doses of 5–10 mg/day, with a cut-off PRU value of 220 for poor responders to clopidogrel without increasing hemorrhagic complications and reducing TECs, as in the two recent studies.23 24 Drawbacks of this work include the absence of a randomized controlled study design as well as the application of add-on therapy such as using cilostazol for the clopidogrel group, particularly for UIAs with stent-assisted embolization. Recent meta-analyses using the abovementioned studies showed that prasugrel was effective in reducing TECs.25 26 Hemorrhagic complications might be influenced by the dose of prasugrel; a high loading dose was associated with a high level of perioperative hemorrhages while a maintenance dose was not associated with delayed hemorrhagic events.25 In our study we achieved successful results using a low dose of prasugrel, the same as previously applied in a Japanese study for coronary intervention,17 without increasing hemorrhagic complications. Most importantly, our study was performed in a prospective manner without the addition of cilostazol in the clopidogrel group, and with a lower maintenance dose than that applied in previous neurointervention studies.
PRU value
The PRU cut-off values with regard to TECs were diverse in previous studies. Early studies used percent inhibition19 20 while subsequent studies used PRU values of 285.22–24 Specifically, Kim et al determined the optimal threshold as 220 by analyzing their historical data and subsequent application in a prospective study.15 Delgado et al reported that a PRU value of <60 or >240 was the strongest independent predictor of perioperative thromboembolic and hemorrhagic complications occurring up to 6 months postoperatively with the Pipeline Embolization Device.11 In our study we adopted PRU cut-off values of <100 and >240, in accordance with these previous studies. A post hoc ROC curve analysis assessing TECs showed that a PRU value of >175.5 was optimal, but with low sensitivity and specificity. The bootstrap AUC-ROC was 0.61 and the average of the thresholds obtained from each of the 10 000 resamples was 180.3. This was nearly the same as that of a recent Japanese report by Nishi et al in which the PRU cut-off for TECs in Japanese patients with clopidogrel was 175 (AUC 0.59, 95% CI 0.44 to 0.75).27
Limitations of study
This was a single-center trial with a limited number of cases. In addition, the tailored group was conducted prospectively during 2017–2019 while the non-tailored group consisted of retrospective data collected from a smaller number of consecutive cases between 2015 and 2017. A prospective randomized controlled study would be desirable in order to more objectively elucidate the efficacy and superiority of prasugrel for neuroendovascular therapy compared with clopidogrel. There was a statistically significant difference in the background characteristics of the tailored and non-tailored groups in terms of the endovascular procedure and device used. This was mainly because of the introduction of flow diverter treatment using Pipeline Flex (Medtronic, Minneapolis, Minnesota, USA) after government approval of its use in Japan in 2017. In general, the complexity and difficulty would be increased in flow diversion therapy compared with other strategies and devices. In contrast, advances in technology and devices would reduce the risk of TECs, as recently reported.28 For example, Wu et al reported that triple platelet therapy, with cilostazol add-on to DAPT, significantly reduced TECs in carotid stenting.29 Unification of devices and strategies would be an important issue in future studies.
Conclusions
The stratified use of thienopyridines in DAPT therapy by tailored administration under PRU monitoring significantly reduced TECs during endovascular therapy for UIAs without increasing hemorrhagic complications. PRU monitoring revealed more stable inhibition of prasugrel compared with clopidogrel.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval was obtained from the institutional Certified Review board (CRB4180003, CR18-102).
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors AS conceived the study and led the research. IN critically revised the manuscript for important intellectual content. AH, KA, MH, YH, TK, YK, TO, KY, AW, JT, KS, SW, TS helped with implementation. SM and YS commented on the paper. TI provided statistical expertise in clinical trial design. All authors contributed to data acquisition, refinement of the study protocol and approved the final manuscript.
Funding This work was supported by grant funding from 2017 Fujita Health University Faculty Research Grant.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.