Article Text
Abstract
Background Patients with large vessel occlusion stroke (LVOS) and a low Alberta Stroke Program Early CT Score (ASPECTS) are often not offered endovascular therapy (ET) as they are thought to have a poor prognosis.
Objective To compare the outcomes of patients with low and high ASPECTS undergoing ET based on baseline infarct volumes.
Methods Review of a prospectively collected endovascular database at a tertiary care center between September 2010 and March 2020. All patients with anterior circulation LVOS and interpretable baseline CT perfusion (CTP) were included. Subjects were divided into groups with low ASPECTS (0–5) and high ASPECTS (6-10) and subsequently into limited and large CTP-core volumes (cerebral blood flow 30% >70 cc). The primary outcome measure was the difference in rates of 90-day good outcome as defined by a modified Rankin Scale (mRS) score of 0 to 2 across groups.
Results 1248 patients fit the inclusion criteria. 125 patients had low ASPECTS, of whom 16 (12.8%) had a large core (LC), whereas 1123 patients presented with high ASPECTS, including 29 (2.6%) patients with a LC. In the category with a low ASPECTS, there was a trend towards lower rates of functional independence (90-day modified Rankin Scale (mRS) score 0-2) in the LC group (18.8% vs 38.9%, p=0.12), which became significant after adjusting for potential confounders in multivariable analysis (aOR=0.12, 95% CI 0.016 to 0.912, p=0.04). Likewise, LC was associated with significantly lower rates of functional independence (31% vs 51.9%, p=0.03; aOR=0.293, 95% CI 0.095 to 0.909, p=0.04) among patients with high ASPECTS.
Conclusions Outcomes may vary significantly in the same ASPECTS category depending on infarct volume. Patients with ASPECTS ≤5 but baseline infarct volumes ≤70 cc may achieve independence in nearly 40% of the cases and thus should not be excluded from treatment.
- thrombectomy
- stroke
- intervention
Data availability statement
Data are available upon reasonable request. The unpublished data from this dataset is held by Grady Memorial Hospital/Emory University and MB/RGN. Requests for data sharing would have to be discussed with them directly.
Statistics from Altmetric.com
Introduction
Current American Heart Association/American Stroke Association guidelines for large vessel occlusion strokes (LVOS) exclude patients with baseline Alberta Stroke Program Early CT Score (ASPECTS) ≤5 from treatment as these patients are thought to have large infarcts and therefore poor prognosis despite endovascular therapy (ET).1 2 However, it has been previously established that the correlation between baseline ASPECTS and infarct volumes is limited.3 Additionally, despite its overall good correlation with outcomes, ASPECTS is not a linear or weighted scale and as such carries broad variations in both tissue volume and regional eloquence across its 10 scored areas.4–6 Therefore, the decision to withhold ET for the entire patient population with low ASPECTS seems to be too arbitrary as it is based on the misguided assumption that ‘all ASPECTS are created equal’.7 8
We hypothesized that patients with low ASPECTS but baseline infarct volumes ≤70 cc would still achieve acceptable rates of good outcomes with ET and sought to study the interaction between baseline core volumes and ASPECTS categories, with special emphasis on the population with low ASPECTS.
Methods
Study population and measures of outcomes
We reviewed our prospectively collected Grady Endovascular Stroke Therapy Outcomes Registry (GESTOR) between September 2010 and March 2020 to identify consecutive patients with anterior circulation LVOS, who underwent non-contrast CT (NCCT) and CT perfusion (CTP) imaging immediately prior to ET. Patients were dichotomized into two baseline ASPECTS groups (1) low ASPECTS (0–5) and (2) high ASPECTS (6-10), and subsequently categorized into limited (≤70 cc) and large (>70 cc) CTP ischemic core volumes. Baseline characteristics, procedural details, and outcome parameters were compared across groups in a two-by-two manner.
The primary outcome measure was the rates of good outcomes defined as functional independence (modified Rankin Scale (mRS) score 0–2) at 90 days. Secondary outcomes included successful reperfusion rates defined as a modified Thrombolysis In Cerebral Ischemia (mTICI) score of 2b/3 and hemicraniectomy rates. Safety endpoints consisted of the rates of any parenchymal hematoma (PH), according to the European Cooperative Acute Stroke Study (ECASS) criteria, and 90-day mortality.9 The last observation carried forward was used for missing final scores on the mRS. This study was approved by the local institutional review board.
Imaging protocol/CTP parameters
All patients included underwent an institutional imaging protocol, including NCCT±CT angiography and CTP. Imaging acquisition parameters were uniform for all patients included in the study. Baseline NCCT was used to determine CT ASPECTS by two experienced vascular neurologists. In cases where there was discordance, the scans were re-adjudicated to reach consensus. CTP was evaluated by fully automated software (RAPID version 4.5.0, iSchemaView, Menlo Park, California, USA). The ischemic tissue volume (ischemic core) was defined by a voxel relative cerebral blood flow of <30% of the normal tissues. The total hypoperfused volume was defined by >6 s delay in the time-to-maximum of the tissue residue function (Tmax), and a penumbral volume of at-risk tissue defined by the difference between total hypoperfused and ischemic core tissue estimates.10
Final infarct volume calculations
Follow-up MRI scans were obtained preferably within the first 72 hours after ET and were performed on a 1.5 T Magnetom Aera (Siemens, Erlangen, Germany). Images were subsequently transferred to a separate workstation for analysis and calculation of full infarct volume (FIV). FIV was semi-automatically measured in the DWI (b-value 1000) sequence or fluid-attenuated inversion recovery (FLAIR) sequence, if more than 72 hours after the procedure, by dedicated stroke research personnel using the Voxel Volume plugin (developed by Soren Christensen, Stanford, 2016) for Osirix 64-bit (Pixmeo, Geneva, Switzerland). Variable window width and center level settings were used for optimal detection and delineation of hyperintensities within the DWI/FLAIR sequences. Where MRI was not available, follow-up NCCT was used to calculate final infarct volumes. Edema producing sulcal effacement was not excluded. Hemorrhagic transformation was incorporated in the FIV whenever present.
Statistical analysis
The Shapiro-Wilk test was used to assess the normality of the variables. Continuous variables were reported as mean±SD if normally distributed or median (IQR) if non-parametric. Categorical variables were reported as proportions. Between groups, comparisons for continuous/ordinal variables were made with Student t-test, Mann-Whitney U test, analysis of variance, as appropriate. Categorical variables were compared by Chi-square test and Fisher exact test, as appropriate. Pairwise deletion was used to handle missing data. Multivariate logistic regression analyses for predictors of good outcomes were performed for variables at the 0.1 level of significance on univariate analysis. Significance was set at p<0.05, and all p values were two sided. Statistical analysis was performed using IBM SPSS Statistics 26 (IBM-Armonk, New York, USA).
Results
Over the study period, 1248 patient fit inclusion criteria, including 125 patients with low ASPECTS (0–5), of whom 16 (12.8%) had large infarct volume, and 1123 with high ASPECTS (6-10), of whom 29 (2.6%) had large infarct volume (figure 1). Significant disagreement between CTP core and ASPECTS categories was found in classifying patients according to their stroke burden ((high ASPECTS vs low ASPECTS) vs ((high infarct volume vs low infarct volume), p<0.001; table 1, online supplemental table 1).
Supplemental material
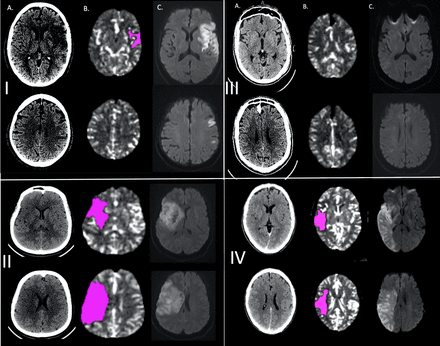
Illustrative cases. (I) Low ASPECTS/low core: older woman, ASPECTS 5, CTP core volume 13 cc, reperfusion TICI 2c, 90-day mRS score 1. (II) Low ASPECTS/high core: middle-aged women, ASPECTS 5, CTP core volume 97 cc, reperfusion TICI 3, 90-day mRS score 4. (III) High ASPECTS/low core: older man, ASPECTS 10, CTP core volume 0 cc, reperfusion TICI 3, 90-day mRS score 0. (IV) High ASPECTS/high core: older man, ASPECTS 10, CTP core volume 76.8 cc, reperfusion TICI 2b, 90-day mRS score 6. (A) Non-contrast CT; (B) CTP (relative cerebral blood flow <30%); (C) DWI-MRI. ASPECTS, Alberta Stroke Program Early CT Score; CTP, CT perfusion; mRS, modified Rankin Scale; TICI, Thrombolysis in Cerebral Infarction.
Agreement between Alberta Stroke Program Early CT Score (ASPECTS) and CT perfusion core volume categories
Population with low ASPECTS
Patients with large infarct volume were younger (41 (37-55) vs 59 (47-70), p=0.008) and had shorter time to treatment (269 (166–348) min vs 452 (293–778) min, p=0.02). There was also a non-statistically significant trend towards higher National Institutes of Health Stroke Scale (NIHSS) and lower rates of atrial fibrillation in the large infarct volume group. Otherwise, baseline characteristics were comparable between groups (table 2).
Baseline characteristics: CT perfusion core versus ASPECTS
For outcomes, there were no differences in rates of successful reperfusion (mTICI 2b/3, 93.8% vs 95.4%, p=0.57 or 90-day mortality, 25% vs 17.6%, p=0.5). However, patients with a large infarct volume tended to have higher rates of hemorrhagic transformation (any PH, 31.3% vs 12.8%, p=0.07), hemicraniectomy (37.5% vs 3.7%, p<0.001), and had large final infarct volumes (164.2 mL (127–238.2) vs 86.3 (43.9–147), p<0.004). A non-significant trend was seen towards worse outcome at 90 days in the large core (LC) group (mRS score 0–2, 18.8% vs 38.9%, p=0.12; table 3). This became significant on multivariate analysis after adjusting for potential confounders (aOR=0.12, 95% CI 0.016 to 0.912, p=0.04; online supplemental table 2)
Outcome measures
Population with high ASPECTS
Patients with large infarct volume were younger (55 (45–62) vs 66 (65–76), p=0.03) and had a trend towards higher baseline NIHSS scores (20 (15–23) vs 16 (11.5–20), p=0.06). Otherwise, baseline characteristics were comparable between groups (table 2).
No differences between groups were seen for procedural and clinical outcomes, including rates of successful reperfusion (100% vs 95%, p=0.4), any PH (6.9% vs 7.2%, p=1.00) and 90-day mortality (13.8% vs 13.5%, p=1.00). Patients with large infarct volume had significantly lower rates of good outcome at 90 days (mRS score 0–2, 31% vs 51.9%, p=0.03) and larger final infarct volumes (73.7 mL (33.2–136.2) vs 23 (9.8–53.2), p=0.01; table 3). On multivariate analysis, large infarct volume was associated with worse outcomes (aOR=0.293, 95% CI 0.095 to 0.909, p=0.04; online supplemental table 2).
Other comparisons between groups
There was no significant difference in the outcomes of patients with low ASPECTS and low infarct volume versus those with high ASPECTS and high infarct volume (90-day mRS score 0–2, 38.9% vs 31%, p=0.44) or patients with low ASPECTS high infarct volume versus high ASPECTS high infarct volume (90-day mRS score 0–2, 18.8% vs 31%, p=0.37). Conversely, patients with low ASPECTS and low infarct volume had worse outcomes than those with high ASPECTS low infarct volume (90-day mRS score 0–2, 38.9% vs 51.9%, p=0.01). Similarly, patients with low ASPECTS and high infarct volume had significant lower chances of achieving good outcomes than those with high ASPECTS low infarct volume (90-day mRS score 0–2: 18.8% vs 51.9%, p=0.009).
Predictors of 90-day good outcomes
In the overall cohort, after including both infarct volumes and ASPECTS in the multivariate predictive model, lower age (aOR=0.958, 95% CI 0.947 to 0.97, p<0.0001), lower baseline NIHSS score (aOR=0.896, 95% CI 0.873 to 0.92, p<0.0001), shorter time to treatment (aOR=0.999, 95% CI 0.999 to 0.999, p<0.0001), and both higher ASPECTS (aOR=2.112, 95% CI 1.254 to 3.556, p<0.005), and lower baseline infarct volume (aOR=3.996, 95% CI 1.536 to 10.395, p<0.005), were found to be independently associated with good outcomes at 90 days.
Discussion
ET is now considered the ‘gold standard’ in the treatment of LVOS presenting in the early and late therapeutic windows,1 11–13 and its indications are progressively increasing. Current guidelines, however, have stringent criteria that may lead to the exclusion of many patients who might still, even if to a lesser extent, benefit from treatment.6 The guidelines do not call for the use of advanced imaging (CTP or MRI) to assess infarct volume in the 0–6 hour window but exclude patients with low ASPECTS as they are thought to have large infarcts, which would presumably lead to bad outcomes. Our data confirm that that just a small minority (2.6%) of patients with ASPECTS 6–10 have a large core, and despite that, their outcomes are encouraging.
Over the past few years, accumulating data has indicated that patients with low ASPECTS might still benefit from thrombectomy. The HERMES meta-analysis showed that patients with ASPECTS 3–5 seem to benefit from thrombectomy, with a lower degree of overall functional disability (mRS shift, aOR=2.00, 95% CI 1.16 to 3.46) and a higher degree of functional independence (mRS score 0–2, 31% vs 16%; aOR=4.27, 95% CI 1.62 to 11.25) at 90 days.14 A recent analysis by Broocks et al, showed that patients with an ASPECTS of ≤5 but good collateral score had improved ambulatory outcome (mRS score 0–3) as compared with those with poor collateral status.15 Similarly, multiple retrospective studies have suggested that treating patients with low ASPECTS may result in better functional outcomes and lower mortality at 90 days than in patients who are not reperfused and/or receive medical treatment only.16–20 Notably, Manceau et al found that an ASPECTS >2 was independently associated with favorable outcomes at 90 days (OR=6.93, 95% CI 1.05 to 45.76,; p=0.04).19 In this context, the definition of low ASPECTS may need to be revised in the near future.
Taking a closer look at secondary/safety endpoints, the above referenced studies had less unanimous results. While some showed a reduction in malignant infarctions, and hemicraniectomy rates,17 20 others reported an increase in the rates of symptomatic hemorrhagic transformation.14 These studies are promising and provide preliminary evidence that large core patients might benefit from mechanical thrombectomy. However, it is important to note that patient selection relied only on the ASPECT scoring system and did not include a measurement of ischemic core volumes, and thus we cannot exclude the possibility that it could have included patients with relatively small cores, which might have confounded the results and erroneously provided a hint of benefit for presumed large infarcts.
A recent analysis by Desai et al found that the prevalence of the clinical-core mismatch paradigm used in the DAWN trial decreased with decreasing ASPECTS but was still present in 13% of the patients in the ASPECTS 0–5 group.21 In our study, 87.2% of patients with low ASPECTS (0–5) had an ischemic core ≤70 cc. While our population was enriched by the selection of patients who were still thought to be reasonable candidates for endovascular treatment, our finding that a large subset of patients with low ASPECTS/low infarct volume achieved good outcomes is consistent with the recent literature and also brings a more refined perspective to the concept of low ASPECTS. Indeed, it has been previously shown that infarct core volumes are highly variable within the same ASPECTS strata, and this consequently leads to varying outcomes.22 This was corroborated by Logan et al, who showed similar rates of good outcomes between ≤6 and>6 ASPECTS groups (37% vs 46%, p=0.85).23 It is also important to note that despite its wide availability and ease of use, the ASPECT scoring has several shortcomings, especially its low inter-rater agreement24 and limited ability to reliably predict infarct volume.25 26 Moreover, the scale is not topographically weighted or linear and thus similar scores do not always translate into the same degree of neurological dysfunction.5 27 It is only logical then, not to rely exclusively on ASPECTS when making treatment decisions for poorly studied patient populations.
In our current analysis, we demonstrated that incorporating ischemic core assessment in addition to ASPECTS to inform endovascular thrombectomy treatment decisions, especially for patients with low ASPECTS might lead to refined selection and fewer treatment exclusions. Campbell et al, in an analysis of the HERMES data, showed that CTP ischemic core volume was as independently associated with functional independence and functional improvement.28 In our patients with low ASPECTS, those with a limited infarct volume had better functional outcomes at 90 days and also lower rates of hemorrhagic conversions than those patients with large infarct volume (3.7% vs 37.5%, p<0.0001). This was also true in the group with high ASPECTS. In this context, CTP may help to include patients with ASPECTS <6, a population that has been thus far excluded from treatment. It is important to highlight that a similar assessment might be possible by evaluating the extent and eloquence of the early ischemic changes on NCCT as opposed to relying only on the ASPECTS number.6 Moreover, automated software is now available that can calculate infarct volumes on NCCT.
Our study has several limitations mostly inherent in its retrospective design and small sample size, especially in the large core groups. We did not have control (medical treatment alone) groups that would have allowed us to measure treatment effect sizes. Additionally, our database does not track patients with LVO who did not undergo thrombectomy, and thus data relating to a proportion of treated patients are not available. Treatment decisions followed a pragmatic approach based on the assessment of the local team and patients/families’ preferences rather than a specific selection protocol. This might have led to a selection bias that could have confounded our results but at the same time allowed us to explore the paradigm of treating patients with low ASPECTS more pragmatically. ASPECTS were not adjudicated by an independent core laboratory. Baseline infarct volume were obtained from CTP which is known to both underestimate and overestimate infarct volumes.29 30
However, despite these limitations, we show that baseline ASPECTS alone is not a good discriminator of outcomes after adjustment for infarct volumes. Specifically, we could document a high rate of good outcomes in 109 consecutive patients with ASPECTS 0–5 but cores ≤70 cc, highlighting that the ‘low ASPECTS’ population is quite heterogeneous. This has several implications in clinical practice. Currently, six ongoing trials are evaluating the safety and efficacy of ET in patients with large core strokes: Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial (RESCUE-Japan LIMIT; ClinicalTrials.gov Identifier: NCT03702413), the Thrombectomy for Emergent Salvage of Large Anterior circulation ischemic stroke trial (TESLA; ClinicalTrials.gov Identifier: NCT03805308), the efficacy and safety of ThrombEctomy iN Stroke with extended leSION and extended time window trail (TENSION; ClinicalTrials.gov Identifier: NCT03094715), IN EXTREMIS LArge Stroke Therapy Evaluation (LASTE; ClinicalTrials.gov Identifier: NCT03811769), Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core (ANGEL-ASPECT; ClinicalTrials.gov Identifier: NCT04551664), and the SELECT-2 trial (ClinicalTrials.gov Identifier: NCT03876457). All rely on NCCT or DWI ASPECTS to determine eligibility for treatment with only ANGEL-ASPECT and SELECT-2 including a measurement of core infarct when available (online supplemental table 3). Nonetheless, these could lead to potentially misleading results if they end up including a large proportion of patients with low ASPECTS and limited infarct volumes. Thus, it is critical to understand that low ASPECTS do not necessarily equate to large infarcts, and assessing core volume is paramount to avoid the exclusion of patients who are known, or very likely, to benefit from treatment.
Conclusions
Outcomes may vary significantly in the same ASPECTS category depending on infarct volume. Patients with low ASPECTS but limited baseline infarct volumes may achieve independence in almost 40% of the cases and thus should not be excluded from treatment. Randomized trials evaluating endovascular treatment in the patient population with low ASPECTS must exclude those with low infarct volume from enrolment otherwise their objective of showing a benefit of endovascular reperfusion in ‘large core’ patients is going to be conceptually flawed.
Data availability statement
Data are available upon reasonable request. The unpublished data from this dataset is held by Grady Memorial Hospital/Emory University and MB/RGN. Requests for data sharing would have to be discussed with them directly.
Ethics statements
Patient consent for publication
Ethics approval
ocal institutional review board (IRB)/Emory University IRB.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @bouslamamd, @diogohaussen, @pisanileonardo
MB and CMB contributed equally.
Contributors Study design: MB, CMB, RGN. Data collection, analysis, and interpretation: MB, CMB, DCH, GMR, LP, MRF, RGN. Drafting the original manuscript: MB. Revising the work critically for important intellectual content: MB, CMB, DCH, GMR, LP, MRF, RGN. Final approval: MB, CMB, DCH, GMR, LP, MRF, RGN. Agreement to be accountable for all aspects of the work: MB, CMB, DCH, GMR, LP, MRF, RGN.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests DCH reports consulting fees for advisory roles with Stryker, Cerenovus, Vesalio, and stock options with Viz-AI. MRF reports research funding from Nico Corp, Inc (ENRICH trial). RGN reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Imperative Care, Medtronic, Phenox, Prolong Pharmaceuticals, Stryker Neurovascular, and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-AI, and Perfuze.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.