Article Text
Abstract
Background Self-expanding stents are increasingly being deployed for stent-assisted coiling or flow diversion of intracranial aneurysms. Complications related to stent misbehavior may arise, however, including lack of expansion, device displacement, or parent vessel thrombosis. We present our experience of various stent removal techniques (stentectomy) with a focus on technical and clinical outcomes.
Methods Stentectomy was attempted either with a single device, including the Alligator, Microsnare, or Solitaire, or by combining a Microsnare with a second device. Dual techniques included in this report are the Snare-over-Stentretriever technique we developed using a Microsnare and a Solitaire, and the previously described Loop-and-Snare technique using a Microsnare and a microwire. The technical success and complication rate, as well as the clinical outcome using the mRS were analyzed.
Results Forty-seven stentectomies were attempted in 36 patients treated for 37 aneurysms. Forty-two devices (89.3%) were successfully retrieved. Single-device stentectomy was successful in 34% of cases, compared with 74% with dual-device techniques. Of the 20 patients with a thrombosed parent or efferent vessel, 17 were successfully recanalized using stentectomy. All successful stentectomy patients made a clinically uneventful recovery, except one with a minor postoperative stroke (mRS 1 at discharge). Failed stentectomy was associated with major ischemic stroke in two patients and death in one patient. There were no stentectomy-related vessel perforations or dissections.
Conclusion While various single devices can be used to safely retrieve dysfunctional intracranial self-expandable stents, dual-device techniques are more than twice as effective, according to our experience.
- aneurysm
- stent
- technique
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data is provided in the supplementary file. A supplementary video is also provided which delineates the methods described in the main article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Various complications may occur when using self-expandable intracranial stents for the treatment of intracranial aneurysms such as lack of wall apposition, mis- or displacement after stent delivery, or acute in-stent thrombosis.1–3 Since stents cannot be withdrawn once released, several options may be considered to deal with these complications: in-stent percutaneous transluminal angioplasty (PTA), additional stent placement, or additional antithrombotic medication.1 4 The stent is then left in place with a delayed risk of vessel stenosis or occlusion and cerebral infarction.5 Ideal treatment may be to remove the stent which simultaneously restores the impaired flow and solves the cause for vessel occlusion. However, little is known about the ability to remove stents.
In this retrospective analysis, we aim: to describe the various techniques that we used for stentectomy, including the snare-over-stentretriever (SOS) technique we developed ;6 to investigate the efficiency of stentectomy; and appreciate the clinical outcome.
Material and methods
Data collection
A retrospective review of a prospectively maintained database on the endovascular treatment of intracranial aneurysms between January 2009 and December 2019 showed that attempt of stentectomy after delivery of a self-expandable stent was achieved in 37 aneurysms out of 3341 aneurysms treated by endovascular means (1.1%).
Medical and imaging records were reviewed for demographics (age, sex), aneurysm status (maximum size, location, ruptured or not), type and location of the stent(s), indication for stent retrieval, materials used for the stentectomy, duration of the stentectomy procedure, immediate angiographic results, imaging findings on MR, and clinical outcome.
MRI was scheduled within the first 3 days after the procedure to evaluate potential ischemic events. Clinical evaluation was evaluated using the modified Rankin Scale (mRS) at discharge.
Endovascular procedure
All procedures were performed under general anesthesia in a dedicated biplane angiosuite (Neurostar, Artis Zee or Artis Icono, Siemens, Erlangen, Germany). Patients were premedicated with clopidogrel and aspirin. The stentectomy was done either during the aneurysm coiling procedure or in a second procedure. The patients were fully heparinized (5–10.000 IU). Preventive treatment of vasospasm was systematically achieved with nimodipin, 3 mg diluted in a 1- liter saline flushing solution. Simultaneously, induced hypertension was obtained either with norepinephrine- or dopamine-delivered IV to maintain a systolic blood pressure between 130 and 150 mm Hg. The guiding catheter was either a 7F Envoy (Cordis Neurovascular, Miami Lakes, Fl, USA) or 8F Guider Soft Tip (Boston Scientific, Marlborough, MA).
Techniques used for stentectomy
Single-device stentectomy
The following devices were used: 2 or 3 mm Alligator (Medtronic, Dublin, Ireland), 2 mm or 4 mm Microsnare (Medtronic), and 4×20 mm Solitaire Stent (Solitaire AB or FR, Medtronic). These devices were used in conjunction with a Rebar 18 microcatheter (Medtronic). The principle of handling was similar for all: the unsheathed device was pushed against the stent to grab a proximal strut of the stent. Complete unsheathing was required with the Alligator and the snare, whereas partial unsheathing was used for Solitaire. Resheathing was achieved by pushing on the microcatheter, thus achieving antegrade displacement of the tip of the microcatheter toward the stent, inducing closure of the device on a strut or on a flared end. This maneuver was repeated until anchoring was successful. Progressive traction on the microcatheter while maintaining the device resheathed enabled to first elongate and then extract the stent.
Snare-over-stentriever technique
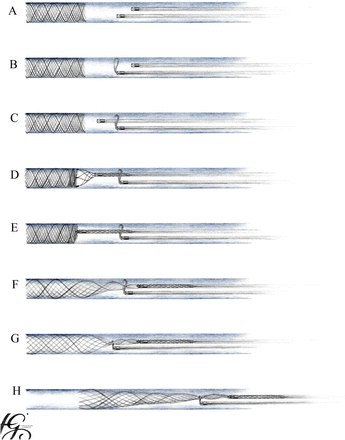
We developed a technique combining a microsnare and a Solitaire stent retriever to achieve a double anchoring in case of failure of the single-device stentectomy.6 This technique is depicted in figure 1. An example is shown in figure 2. For this purpose, a snare was deployed through a first microcatheter proximal to the stent (figure 1-B). A second microcatheter was then navigated through the opened snare (figure 1-C). Anchoring of a strut of the stent was achieved with a Solitaire entered through the second microcatheter (figure 1-D, 1-E). Progressive traction on the stent allowed its elongation and reduction in diameter. The opened snare could then be navigated in the monorail technique alongside the second microcatheter and over the elongated stent (figure 1-F). Once around the stent, retraction on the snare enabled a stable anchoring of the whole stent (figure 1-G) and further stent extraction (figure 1-H).
Schematic view of stent over-snare technique: detail is presented in the text.
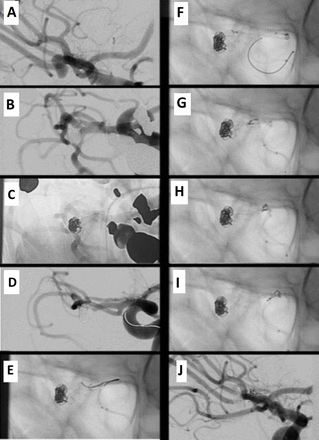
Unruptured aneurysm of the right middle cerebral artery bifurcation. The aneurysm was treated by balloon-assisted coiling followed by placement of a self-expandable baby Leo stent from the inferior MCA division branch to the M1 segment. The patient was clinically asymptomatic at extubation but developed a left-sided hemiparesis and neglect in the following hour. after exclusion of a cerebral hemorrhage on CT, The patient was brought back to the angiosuite. A repeated digital subtraction angiography (DSA) demonstrated an occlusion of the stent and the inferior middle cerebral artrey (MCA) division branch. Stentectomy was achieved using the SNARE over stent technique.
Loop-and-snare technique
This technique was achieved as described in the publications of Barburoglu7 and Parthasarathy.8 A microguidewire was passed through the proximal struts of the stent. A snare then captured the tip of the microguidewire through a second microcatheter. Traction on the loop of the wire allowed removal of the stent.
Ethical considerations
This cohort study complies with the Strengthening the Reporting of Observational Study in Epidemiology (STROBE) statement.
For this retrospective study, all patients consented that their data be anonymized and generally used for future studies. Our institutional review board waived specific consent for the present study.
Results
Retrieval of 47 stents was attempted in 36 patients who were treated for 37 aneurysms. In 28 patients, a single stent was delivered per aneurysm. In eight patients, multiple stents were delivered to achieve T stenting (n=4), kissing stents (n=2), or telescopic stents (n=2). Among 37 treated aneurysms, the indications for stentectomy were in-stent parent vessel thrombosis (n=19), thrombosis of an artery covered by the stent (n=1), deficient stent expansion (n=9), initial misplacement (n=2), or secondary displacement (n=4) and the need to access to an artery bridged by the stent to control a distal hemorrhage (n=1) or to remove a dislocated coil (n=1).
All aneurysms were unruptured and either previously untreated (n=28) or recanalized (n=9). The results are presented as a online supplemental table 1.
Supplemental material
Forty-two out of 47 stents could be retrieved successfully. The stents were braided (n=46) or laser-cut (n=1) and included the Baby Leo (n=19) (Balt, Montmorency, France), Leo (n=10) (Balt), Lvis (n=7) (MicroVention, Tustin, CA, USA), Flow-Redirection Endoluminal Device FRED (n=3) (MicroVention), Silk (n=3) (Balt), Pipeline (n=3) (Medtronic), Derivo (n=1) (Acandis, Pforzheim, Germany), and Acclino (n=1) (Acandis).
The efficiency of each device or technique was respectively Loop-and-Snare 75% (3/4), Snare-over-Stentriever 73% (16/22), Snare 56% (19/34), Solitaire 26% (6/23), and Alligator 0% (0/16).
The median duration for successful stentectomy was 11 min. There was no coil displacement during or after removal of the stent.
Out of 20 patients with a vessel occlusion, successful stentectomy (n=17) led to a minor stroke (mRS=1) in one patient, whereas failed stentectomy (n=3) led to major stroke (mRS=3) in two patients, and death in one patient. In 15 patients with a misplaced, displaced, or unexpanded stent, stentectomy was successful in 13 patients without technical or clinical complications. Failure of stentectomy in two additional patients was handled by an increased medication of anti-aggregants without further consequence. In two patients with bifurcation aneurysms in whom stentectomy was required to get access to the artery covered by the stent in order to control a distal bleeding or remove a migrated coil, the complication could be successfully handled after removal of the stent.
Discussion
Various devices have been proposed for endovascular retrieval of misplaced materials. Since the early 1990s, microsnares have mostly been described in isolated case reports.9–13 Since 2006, the Solitaire stent initially named Solo and intended to be used for the treatment of intracranial aneurysms14 has been increasingly used to retrieve intracranial thrombi in the treatment of acute stroke15 but also to retrieve dislocated coils.16 17 Other case reports also mentioned the use of the Alligator18 (Medtronic), Merci (Concentric Medical Inc., CA, USA),19 20 or other stent retrievers such as the Trevo (Stryker) for the retrieval of coils.21 Currently, only a few case reports mentionthe possibility of retrieving stents.7 8 22–24
Forty-two out of 47 stents could be retrieved after complete release despite the absence of a dedicated device. The high success rate of 89% and the relatively short duration in our experience do not reflect the fact that stentectomy remains a technically challenging procedure where various steps have to be considered:
Anchoring of the stent, which is the ability to catch a strut of the stent is the first requirement. This may be simple to achieve with a snare if the proximal part of the stent is not apposed to the vessel wall or in stents harboring flared ends such as the Lvis or Fred. But in the case of good wall apposition without flared ends such as with the Pipeline, Silk, and Leo, anchoring cannot be achieved. In these situations, we only managed to dislodge the stent from the vessel wall when pushing a partially expanded Solitaire against the proximal end of the stent to grab a strut.
The stability of anchoring is another requirement. Anchoring may be lost after traction is applied on the device. The snare, allowing a strong anchoring, had a higher success rate (56%). The Solitaire with a weak anchoring was only successful in 26%. The Alligator with a high ability but a poor stability of anchoring failed to retrieve the stent in all attempted procedures (0%).
In the case of persisting failure despite repeated attempts with various devices, we developed a dedicated technique combining a microsnare and a Solitaire allowing a 73% success rate as previously reported in various meetings.6 A first anchoring is achieved with a Solitaire in order to allow a second anchoring with a microsnare. With growing experience, we directly used this combination of devices. This allowed a short median duration for stentectomy. This technique was named the SOS technique when later evaluated in-vitro, where the high success rate could be confirmed.25
In four patients, we used the wire-and-snare technique7 also described as loop-and-snare technique8 where a combination of two microcatheters is required. Anchoring is achieved with a microguidewire passed through the struts of the stent after catching its tip with a snare. This technique was successful in three attempts, including two SOS technique failures but failed in one patient where the wire could not cross the struts of a flow diverter. Though the efficiency of the loop-and-snare technique (75%) is very similar to the SOS technique (73%), a conclusion cannot be drawn for the most efficient technique given the low number of patients treated with loop-and-snare.
There were no complications related to stent retrieval, even though some brain vasculature displacement was seen during the retraction maneuvers. Pushing a partially expanded Solitaire against the vessel wall may be potentially traumatic. There was, however, no hemorrhagic complication in our experience.
Displacement of a loop or of the whole cast of coils due to the retrieval of the stent did not occur in any of the patients treated by coiling and stenting (n=27). The stability of the coils may be explained by the systematic use of a remodeling balloon during coiling before delivery of the stent through the dual lumen balloon.
The indication for post-coiling stenting may be raised regarding the high number of successful stentectomies that were achieved in this series. Stenting was intended to stabilize the long-term occlusion as the recanalization rate seems lower for stent-assisted coiling than balloon-assisted coiling.26 It may thus be argued that additional placement of a stent after coiling may not be required and increases the morbidity of the procedure. However, retreatment of recanalized aneurysms is often more challenging than untreated aneurysms due to a more unfavorable neck to sac ratio explaining our choice of balloon-assisted coiling followed by stenting. This combination of techniques allows a 92% occlusion rate 3 years after treatment.27 The technical evolution with the ability to place a stent through a double-lumen remodeling balloon further allows an easy placement of a stent after balloon-assisted coiling. The preferential use of braided stents, when thrombogenicity is higher than laser-cut stents,2 may be another factor explaining the number of in-stent thrombosis in this series. Finally, the allover rate of 1.1% of stent retrieval out of all endovascular procedures for aneurysm treatment in our institution compares favorably to the literature with a 5.9% rate of in-stent thrombosis for unruptured intracranial aneurysms.2
In patients with a vessel occlusion (n=20), the clinical outcome was dependent on the ability to retrieve the stent. Stentectomy allowed a good clinical outcome in all patients where it was successful (n=17), the mRS was 0 in 16 patients and one in one patient. Factors contributing to a good clinical outcome may also have been a short delay in settling the diagnosis of stent occlusion and a short median duration of stentectomy. In three patients where we failed to retrieve the stent, the outcome was poor. Anti-GPIIbIIIa did not either allow to recanalize the stent (n=2) or could not be administered due to extensive ischemia that already occurred at time of diagnosis (n=1).
The ability to retrieve stents raises the question of how to treat acute stent thrombosis. Several other options may be proposed:
Medical treatment with anti-GPIIbIIIa anti-aggregants is an efficient way to proceed with a reported rate of neurologic complications after in-stent thrombosis of 27%.2 Stent manipulation is thus avoided but the delay until a recanalization can be achieved is variable. The recanalization may, however, only be partial which may not prevent a stroke to occur. Besides, the allover level of anti-aggregation is increased with a potential increased hemorrhagic risk.28
An in-stent PTA is another possible rescue technique to restore flow through an occluded stent. The intraluminal thrombus may limit the ability to catheterize through the stent. A PTA may also increase the risk of perforator or side-branch occlusion.
Additional stent placement through the thrombosed stent may increase the degree of expansion of the first stent and enable restoral flow. However, it may be challenging to assess whether the second stent is strictly within the lumen of the first stent as intended. If the second stent is crossing the struts of the first stent, expansion of the second stent will only be partial, which may rather potentialize further thrombogenicity.
The advantage of stentectomy is to restore normal flow as soon as the stent is withdrawn. It also clears the cause of the vessel occlusion which is the stent itself. Besides, anti-aggregation can be stopped. If the stent is left in place, anti-aggregation must be maintained, which carries a hemorrhagic risk in case of acute stroke.
The ability to retrieve stents raises the question of whether unexpanded stents should be left in place or rather be retrieved. Indication may depend on the degree of stent expansion, which can be appreciated on fluoroscopy or cone beam CT. In this series, stent retrieval was attempted in 10 stents with missing wall apposition after failure of in-stent balloon PTA. Additional eight stentectomies were achieved for displaced or misplaced stents. Seventeen out of these 18 stents could successfully be retrieved without complications. If left in place, such unexpanded stents may explain the increased risk of delayed thromboembolic events as previously described in stent-assisted coiling of unruptured aneurysms5 with the need for an increased or prolonged medication with anti-aggregants. Besides, unexpanded, misplaced, or displaced stents may be an obstacle in further treatment with a flow-diverting stent. The good results of stentectomy led us therefore to enlarge its indications to inadequate stent expansion or placement.
Our study has several limitations. Besides its single-institution retrospective nature and inherent biases, one of the devices presented here is no longer commercially available. Since this device never allowed a successful stentectomy, this does not interfere with the results. The lack of a control group where all techniques excluding a stentectomy would have been performed would have enabled us to define which technique is preferable. All stents except one in this study were braided stents, which reflects our current practice. Although we presume that the various techniques presented here may be effective for the retrieval of laser-cut stents, we cannot give a definite statement. Finally, the reproducibility of these techniques, although confirmed in vitro, requires a minimum of experience and training in these bailout situations.
Conclusion
Various techniques can be used to remove stents safely. The SOS or loop-and-snare stentectomy are the most efficient according to our experience.
Supplementary video
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data is provided in the supplementary file. A supplementary video is also provided which delineates the methods described in the main article.
Ethics statements
Patient consent for publication
Ethics approval
The review board waived the need for specific patient informed consent due to the retrospective nature of the study.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors RC: study concept, overall supervision, writing of the manuscript. PCS: gathering of information, a systemic search of the literature. MW: gathering of information, a systemic search of the literature. RR: gathering of information, a systemic search of literature, statistical analysis. EY: statistical analysis, systemic search of literature. PJM: study concept, final edit of the manuscript, writing of the manuscript
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests René Chapot received consulting fee or speaker honoraria from Balt, Microvention, Rapid Medical, Siemens, and Stryker. Pascal Mosimann received public funding related to intracranial aneurysm and vasospasm therapy from the Swiss National Science Foundation and the Innosuisse agency. Reza Rikhtegar received a travel grant from Boehringer Ingelheim and Microvention.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.