Article Text
Abstract
Background In contrast to conventional CT perfusion (CTP) imaging, flat panel detector CT perfusion (FD-CTP) imaging can be acquired directly in the angiosuite.
Objective To evaluate time-resolved whole brain FD-CTP imaging and assess clinically important qualitative and quantitative perfusion parameters in correlation with previously acquired conventional CTP using the new RAPID for ANGIO software.
Methods We included patients with internal carotid artery occlusions and M1 or M2 occlusions from six centers. All patients underwent mechanical thrombectomy (MT) with preinterventional conventional CTP and FD-CTP imaging. Quantitative performance was determined by comparing volumes of infarct core, penumbral tissue, and mismatch. Eligibility for MT according to the perfusion imaging criteria of DEFUSE 3 was determined for each case from both conventional CTP and FD-CTP imaging.
Results A total of 20 patients were included in the final analysis. Conventional relative cerebral blood flow (rCBF) <30% and FD-CTP rCBF <45% showed good correlation (R2=0.84). Comparisons of conventional CTP Tmax >6 s versus FD-CTP Tmax >6 s and CTP mismatch versus FD-CTP mismatch showed more variability (R2=0.57, and R2=0.33, respectively). Based on FD-CTP, 16/20 (80%) patients met the inclusion criteria for MT according to the DEFUSE 3 perfusion criteria, in contrast to 18/20 (90%) patients based on conventional CTP. The vessel occlusion could be correctly extrapolated from the hypoperfusion in 18/20 cases (90%).
Conclusions In our multicenter study, time-resolved whole brain FD-CTP was technically feasible, and qualitative and quantitative perfusion results correlated with those obtained with conventional CTP.
- Stroke
- CT perfusion
- Thrombectomy
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
Introduction
In patients with acute ischemic stroke, perfusion imaging facilitates detection of the occluded vessels,1 2 influences decision-making regarding therapy options, and is recommended especially in delayed time windows.3 4 Perfusion imaging helps to delineate infarct core and hypoperfused, but not yet infarcted, tissue (tissue at risk, penumbra), with generally accepted thresholds of relative cerebral blood flow (rCBF) <30% and time to maximum (Tmax) >6 s.5
In contrast to conventional CT perfusion (CTP), flat panel detector CT can be acquired directly in the angiosuite and can generate angiograms (FD-CTA) and perfusion maps (FD-CTP) directly on the intervention-table. This allows direct transport of patients to the angiosuite (‘direct to the angio’ workflow), reduces time to puncture, and increases the likelihood of clinical improvement.6–11 It is an essential precondition that FD-CTP provides comparable data to that obtained from validated conventional CTP.
Until recently, FD-CTP was limited to the acquisition of parenchymal cerebral blood volume or FD-CBV maps using a two-sweep C-arm rotation protocol lacking the temporal resolution needed for time-resolved dynamic perfusion imaging such as CBF or Tmax.12 Many studies have investigated dynamic perfusion imaging in the angiosuite.13 14 The recent clinical introduction of a multiphase FD perfusion acquisition protocol (syngo DynaCT Multiphase, Siemens Healthineers AG) enables the acquisition of time-resolved whole brain FD-CTP datasets that overcome this technical limitation. RAPID for ANGIO (RAPID, iSchemaView, USA) is the first, and currently only, clinically available postprocessing software for multiphase FD-CTP acquisitions based on the RAPID software.6
To evaluate FD-CTP imaging, we assessed clinically important qualitative and quantitative perfusion parameters in correlation to prior acquired conventional multislice CTP using the new RAPID for ANGIO software.
Methods
Patient population
We included patients from six centers: San Francisco General Hospital covered by the University of California San Francisco (San Francisco, USA), University Hospital of Bern (Switzerland), Kantonsspital St Gallen (Switzerland), University Medical Center Göttingen (Germany), Advocate Lutheran General Hospital (Chicago, USA), and University Hospital of Basel (Switzerland) from October 2016 to February 2021. All patients underwent mechanical thrombectomy (MT), had preinterventional FD-CTP imaging and met the following inclusion criteria:
Intracranial internal carotid artery, M1, or M2 occlusion
No complete or partial recanalization between the scans (eg, M1 occlusion in the initial scan and M2 occlusion in the FD-CT scan)
Technically adequate baseline conventional CTP and FD-CTP imaging without serious motion or imaging artifacts
A maximum of 120 min delay between conventional CTP and FD-CTP
Approval for this multicenter study was obtained from the institutional review board. The requirement for informed consent was waived owing to the retrospective nature of the study.
Imaging protocol
Workflow
After clinical examination by a neurologist at the emergency department, patients underwent conventional stroke CT imaging, including CT angiography (CTA) and CTP. If the eligibility criteria for MT were fulfilled, patients were either transferred to a thrombectomy center (transfer patients) or directly to the angiosuite (mothership patients) and received FD-CT imaging, including FD-CTP, preinterventionally at the discretion of the treating physician. The decision whether to perform FD-CT imaging was based on clinical reasoning and provided additional valuable information that allowed the involved physicians to adjust the treatment strategy accordingly.
FD-CTP acquisition
Biplane flat panel detector angiographic systems (ARTIS Icono and Artis Q, Siemens Healthineers AG, Forchheim, Germany) were used to acquire imaging data for the whole brain. Ten rotational sweeps (with a duration of 5 s each and a turnaround of about 1 s) of the angiographic C-arm system around the patient were performed (the multi-sweep acquisition on Artis Q is based on a research prototype). Z-axis coverage of the FD-CTP was full brain. The first two rotations served as mask runs, whereas the following eight rotations recorded inflow and outflow of contrast agent, creating time–density curves with eight time points (online supplemental figure I). Injection of contrast agent (60 mL Iopamiro 400 or 300 or 70 mL Omnipaque 350) was started at the same time as the mask run via an 18 G left or right cubital venous line and was followed by a chaser of 40–60 mL saline flush (mono or dual-head power injector at an injection rate of 5 mL/s).
Supplemental material
CTP and FD-CTP postprocessing
Pos-processing of conventional CTP and FD-CTP scans was performed using dedicated software based on RAPID (RAPID for ANGIO, iSchemaView). Reconstruction of the CTP and FD-CTP datasets was performed on the vendor’s product software and then transferred to RAPID and RAPID for ANGIO for further processing. The default threshold for infarct core used for conventional CTP using RAPID is rCBF <30% and for FD-CTP using the RAPID for ANGIO software rCBF <45%. The rationale behind using a different threshold for FD-CTP was based on phantom data, which showed that FD-CTP is less sensitive than conventional CTP for detecting CBF values below 30 mL/100 g/min. The phantom data are presented in online supplemental figure II. In addition, a pilot study using the <45% threshold for FD-CTP in patients with acute stroke demonstrated a strong correlation with conventional CTP (Pearson=0.91, Spearman=0.87) with an intraclass correlation coefficient of 0.89 (95% CI 0.67 to 0.96).13
Outcome
Quantitative performance was determined by comparing volumes of infarct core (defined as rCBF <30% for conventional CTP using RAPID and <45% for FD-CTP using RAPID for ANGIO), penumbral tissue (defined as Tmax >6 s for both modalities), and mismatch (penumbral tissue (mL) – infarct core (mL)). In addition, the influence of time between the scans was evaluated continuously and for different groups (<30 min, 30–60 min, and >60 min) and depicted graphically.
Eligibility for MT according to the perfusion imaging criteria of DEFUSE 3 was determined for each patient and for both modalities (infarct core <70 mL, mismatch volume ≥15 mL, and mismatch ratio ≥1.8).15
Statistical analysis
Baseline characteristics of the groups were compared using the Kruskal-Wallis test for continuous variables or Fisher’s exact test for categorical variables. Data are displayed as median (IQR) and n (%) if not otherwise specified.
Correlation of baseline CTP volumes (rCBF <30%, Tmax >6 s, mismatch) with FD-CTP volumes (rCBF <45%, Tmax >6 s, mismatch), stratified by the time between the scans, was graphically depicted in scatter plots and R2 (coefficient of determination) was calculated. Bland-Altman analysis was conducted using the ‘BlandAltmanLeh’ (version 0.3.1) package for R. The x-axis of the Bland-Altman plots displays the mean of the two measurements (volume measured by CTP and by FD-CTP), and the y-axis shows the difference between the two measurements.
A two-tailed p value of <0.05 was considered statistically significant. Statistical analysis was performed using R (version 4.0.2).16
Results
Of 29 patients with adequate conventional CTP and FD-CTP, two had to be excluded owing to reperfusion between the scans, two owing to vessel occlusions of the posterior circulation, two because the time limit between the scans (>2 hours) was exceeded, and three patients received magnetic resonance perfusion as baseline imaging, leaving a final population of 20 (online supplemental figure III). Of these 20 patients, the majority were treated in Bern (12 patients). Baseline characteristics are shown in table 1.
Patient characteristics at baseline
Quantitative correlation FD-CTP versus conventional CTP
Conventional rCBF <30% and FD-CTP rCBF <45% generally showed good correlation (R2=0.84, figure 1A). Underestimation of infarct core in FD-CTP occurred in two cases and infarct core was missed in one case, all with low volumes of infarct core (<50 mL). Conventional CTP Tmax >6 s versus FD-CTP Tmax >6 s (figure 1B) and CTP mismatch versus FD-CTP mismatch (figure 1C) showed more variability and a lower correlation (R2=0.57, and R2=0.33, respectively).
Comparison of different perfusion volumes between conventional CT perfusion (CTP) and flat panel detector perfusion (FD-CTP), stratified by times between the scans. (A) For relative cerebral blood flow (rCBF; R2=0.84), (B) for Tmax (R2=0.57), and (C) for mismatch (R2=0.33). Regression lines and 95% CI are displayed.
Bland-Altman analysis for rCBF showed a bias of −11.7 mL (95% CI −23.7 to 0.4 mL) but had the smallest range of agreement levels of −62.1 to 38.8 mL (figure 2A). FD-CTP Tmax >6 s was also lower on average than Tmax >6 s derived from CTP (bias=−19.5 mL, 95% CI −48.7 to 9.7 mL; agreement levels −141.8 to 102.8 mL) (figure 2B). However, agreement on mismatch between the two modalities showed the smallest bias of −7.9 mL (95% CI −36.3 to 20.64 mL) and agreement levels of −127.2 to 111.5 mL (figure 2C).
Bland-Altman plots showing the differences in perfusion volumes obtained with conventional CT perfusion (CTP) and flat panel detector perfusion (FD-CTP) (A) for relative cerebral blood flow (rCBF), (B) for Tmax, and (C) for mismatch. The mean difference (bias, continuous line) and 95% CI of agreement (dashed lines) are displayed.
Overall, the differences in perfusion volumes between CTP and FD-CTP did not change with increasing time between the scans (online supplemental figure IV).
Based on FD-CTP, 16/20 (80%) patients met the inclusion criteria for MT according to the DEFUSE 3 perfusion criteria, in contrast to 18/20 (90%) patients based on conventional CTP. The reasons for exclusion based on FD-CTP were infarct core >70 mL (n=3) and mismatch ratio of <1.8 (n=2), and, based on conventional CTP, infarct core >70 mL (n=2) and mismatch ratio <1.8 (n=1).
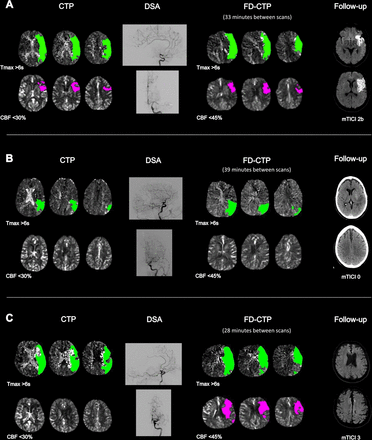
Illustrative examples are shown in figure 3.
(A) M1 occlusion left, shown in the digital subtraction angiography (DSA), with corresponding hypoperfusion patterns in CT perfusion (CTP) and in flat panel detector perfusion (FD-CTP). After modified Thrombolysis in Cerebral Infarction (mTICI) 2b was achieved, the first follow-up diffusion-weighted imaging (DWI) showed infarction of the anterior portion of the left M1 territory. (B) M2 occlusion left, as shown in the DSA scan, with corresponding CTP and FD-CTP hypoperfusion patterns. Follow-up CT showed complete infarction of the affected M2 territory after mTICI 0. (C) M1 occlusion left with corresponding CTP and FD-CTP hypoperfusion patterns and corresponding capillary blush deficit in the DSA scan (28 min between scans). The increase between CTP and FD-CTP may indicate rapid infarct progression but follow-up DWI showed no infarction, probably due to rapid and complete reperfusion (mTICI 3). CBF, cerebral blood flow.
Discussion
This exploratory study has the following main findings: (1) time-resolved whole brain FD-CTP is technically feasible, (2) overall, quantitative FD-CTP maps showed good agreement and correlated well with conventional CTP maps, and (3) identification of MT-eligible patients according to the DEFUSE 3 criteria was feasible in most cases.
The Bland-Altman analyses showed that, on average, volumes of tissue with rCBF <45%, Tmax >6 s, and mismatch were larger in FD-CTP than when derived from conventional CTP (with rCBF <30%, respectively). This could be due to measurement error or because, despite the relatively short time between the scans, infarct core and tissue with Tmax >6 s tend to become larger with longer duration of vessel occlusion. Furthermore, we saw no signs of systematic errors or biases in the Bland-Altman plots as the differences showed a tendency to increase with larger average measurements (in the sense of a random relative error) and our biases were even smaller than those reported in a similar single-center study comparing rCBF and Tmax from CTP and FD-CTP.13
Although measurement of penumbra volumes showed a smaller correlation between the two scans, mismatch volume showed the smallest mean error in the Bland-Altman analyses. Also, there was agreement in every case regarding the presence or absence of a favorable mismatch profile (ie, penumbral tissue) and only a few patients who would not have been eligible for MT according to the perfusion imaging criteria of DEFUSE 3, where penumbra volume plays a key role. Furthermore, depiction of the infarct core and penumbra worked well and determination of the occlusion site was reasonably straightforward. This is particularly important from a clinical standpoint and helps in the decision-making for diagnosis and choice of therapy.
Previous work comparing conventional CTP and FD-CTP mainly examined CBV as another perfusion parameter, with values of CBV <2 mL/100 g indicating infarct core.17 Fiorella et al reported 100% sensitivity and 81.3% specificity for detecting any CBV deficit.18 Other studies reported a correlation coefficient of R2=0.79 when comparing six regions of interest (ROIs)19 and R2=0.85 when comparing CBV and CBF ROIs directly in the perfusion deficit.20 Immediately after MT, correlation of ROIs of FD-CTP CBV and follow-up CTP CBV was even better (R2=0.9).21 Given that CBV can be calculated as CBF ×mean transit time22 and is therefore related to CBF, our findings are in line with these results and further strengthen the viewpoint that FD-CTP is clinically applicable.
To the best of our knowledge, the present multicenter study evaluated the largest cohort to date of patients with acute ischemic stroke undergoing time-resolved whole brain FD-CTP. Our results reiterate the validity of technically adequate FD-CTP using the first commercially available software as compared with conventional CTP.
Our results also support the establishment and further evaluation of direct-to-angio approaches in everyday clinical practice, as FD-CT can produce FD-CTP as well as soft tissue brain imaging and multiphase FD-CT angiography, similar to conventional CT ‘stroke protocol’ sequences. This, in turn, can speed up in-hospital workflows and may ultimately increase the chances of good clinical outcome.6–11 Of paramount importance to the success of the direct-to-angio workflow is the familiarity of the technical and nursing staff as well as the diagnostic neuroradiologists with the practice. Understanding how to effectively use the angiosuite as a conventional CT scanner and interpret its imaging output will be central to translating trials into improved general practice.23
The use of FD-CTP post-thrombectomy is another possible application and the next frontier where FD-CTP could add significant value. In cases of incomplete thrombectomy with residual occlusion—for example, the decision whether to perform rescue maneuvers (eg, with distal stent retrievers24 25 or administration of a thrombolytic drug26 27) or to stop, is currently open to discussion. Intraprocedural magnetic resonance may also aid in such real-time treatment decision-making,28 although FD-CT is considerably simpler from a workflow standpoint. FD-CTP could delineate penumbra and infarcted tissue and help to prevent futile (no more salvable brain tissue) or unnecessary angiographic improvement (no infarction would have developed if no rescue maneuver had been performed).29
Limitations
We selected only patients with vessel occlusions in the intracranial internal carotid artery, M1 or M2 segments and further validation for occlusions of the posterior circulation and of peripheral occlusions is warranted. Also, our study had a relatively small sample size. To overcome these limitations, studies including larger populations would be needed. FD-CTP is derived from fewer time points than CTP and this could potentially influence the resulting perfusion calculations. Finally, all patients had recently received a bolus of intravenous contrast. One report has suggested that contemporaneous contrast administration may affect core estimation.30
Conclusions
In our multicenter study, time-resolved whole brain FD-CTP was technically feasible, and qualitative and overall quantitative perfusion results correlated well with those obtained using conventional CTP. This suggests that establishment of a direct-to-angio approach is feasible and may increase the chances of good clinical outcome. Application of FD-CTP post-thrombectomy could be the next frontier where FD-CTP could add significant value by helping in decision-making in cases of incomplete thrombectomy.
Supplemental material
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @chris_kurmann
Correction notice This article has been corrected since it was first published. The open access licence has been updated to CC BY.1 7th May 2023.
Contributors CCK contributed to concept and design, data acquisition, analysis and interpretation of data, and writing of the initial draft. JK and PM contributed to concept and design, data acquisition, interpretation of data, revision of the manuscript for important intellectual content, and supervision. PM acted as the guarantor. All other authors contributed to concept and design, data acquisition, interpretation of data, and revision of the manuscript for important intellectual content.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests PM reports receipt of research support from Siemens, Cerenovus, Medtronic, Stryker, the Swiss Heart Foundation. and the Swiss National Foundation; receipt of consultant fees from Medtronic, Cerenovus, Phenox, and Microvention, unrelated to the submitted work. DLC reports funding by a Siemens Healthineers-Grant. MP reports research collaboration with Siemens. DKL is a member of the advisory board of Siemens and honoraria/consultant for RAPID. AI, Stryker, Medtronic, Siemens, Phenox, Asahi. He reports stock options of Three Rivers, QAPEL, Methinks, Bendit, Synchron, Vastrax, NTI, VizAI, ELUM. GWA is a consultant for Gentech and is a consultant for, and has equity interests in, iSchemaView. JK reports grants from the Swiss Academy of Medical Sciences/Bangerter Foundation, Swiss Stroke Society, and Clinical Trials Unit Bern during the conduct of the study.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.