Abstract
Background
Experience with the endovascular treatment of cerebral aneurysms by the Flow Re-Direction Endoluminal Device (FRED) is still limited. The aim of this study is to discuss the results and complications of this new flow diverter device (FDD).
Methods
Between November 2013 and April 2015, 20 patients (15 female and five male) harboring 24 cerebral aneurysms were treated with FRED FDD in a single center.
Results
Complete occlusion was obtained in 20/24 aneurysms (83 %) and partial occlusion in four (17 %). Intraprocedural technical complication occurred in one case (4 %) and post-procedural complications in three (12 %). None reported neurological deficits (mRS = 0). All FRED were patent at follow-up. No early or delayed aneurysm rupture, no subarachnoid (SAH) or intraparenchymal hemorrhage (IPH) no ischemic complications and no deaths occurred.
Conclusions
Endovascular treatment with FRED FDD is a safe treatment for unruptured cerebral aneurysms, resulting in a high rate of occlusion. The FRED is substantially equivalent to the other known FDDs, which show similar functions and technical profiles.
Similar content being viewed by others
Introduction
Flow diverter devices (FDDs) are rapidly becoming a suitable and, in selected cases, preferred alternative to traditional endosaccular treatment with coils. These new devices consist of a high-attenuation braided mesh stent placed in the parent artery at the level of the neck to disrupt the intra-aneurysmal flow and subsequently create intra-aneurysmal thrombosis; the exposed surface of the FDD is also good support for the development of the neointima [16].
FDDs have shown huge advantages for difficult-to-treat aneurysms such as wide-necked, fusiform, or giant aneurysms, or those with complex morphology [1, 5–9, 14, 18–24, 27–31]. Although the introduction of this device is relatively recent, the experience is rapidly increasing and large studies have been reported. However, the choice of the best endovascular procedure and the indications to the use of FDDs are still a matter of debate.
Five types of FDDs have been approved for the treatment of intracranial aneurysms: the Pipeline Flex Embolization Device (PED) (Covidien, Mansfield, MA, USA), the Silk (Balt Extrusion, Montmorency, France), the FRED (Microvention, Tustin, CA, USA), the P64 Flow-Modulation Device (Phenox) and the Surpass (Surpass; Stryker Neurovascular, Fremont, CA, USA). Almost all studies report experiences with PED and Silk [1, 6–10, 14, 18, 19, 21–24, 26–28, 31]; on the other hand, the experience with new devices as FRED [12, 17, 20, 25] or p64 [4, 13] is still limited.
We report a series of 20 patients harboring 24 aneurysms treated by the flow re-direction endoluminal device (FRED) (Microvention, Tustin, CA, USA).
The aim of the study is to discuss the results and complications of this new FDD.
Materials and methods
Study design
This study was designed to define the role of the (FRED) in the endovascular treatment of unruptured cerebral aneurysms.
We retrospectively reviewed all cases of cerebral aneurysms that were admitted to the Division of Neurosurgery of the Federico II University of Naples between November 2013 and April 2015. We enrolled 20 patients in the study harboring 24 cerebral aneurysms treated with FRED FDD.
Inclusion criteria were no previous neurosurgical treatment, patient consensus to the endovascular procedure, aneurysms difficult to treat with other techniques (neurosurgical and endovascular) because of the anatomical configuration. All patients had complete clinical-radiological pre- and post-procedural data.
The radiological definition was obtained in all cases by computed tomography angiography (CTA), with the native images and the 2D multi-planar reconstructions (MPR), and by digital subtraction angiography (DSA). The aneurysm location, size, neck, dome-to-neck ratio were considered.
The endpoints were angiographic evidence of complete aneurysm occlusion, recanalization rate, occlusion of the parent artery, and clinical and radiological evidence of brain ischemia.
The occlusion rate was evaluated according to the O’Kelly-Marotta Scale (OKM) for flow diversion based on the degree of filling (A total filling, B subtotal filling, C entry remnant, D no filling) [22].
Patient characteristics
The 20 patients were 15 females and five males, ranging in age between 32 and 70 years (median age, 52 years). All had unruptured incidental aneurysms, with no previous subarachnoid or cerebral hemorrhage.
Eighteen patients had a single device, while two had two FDD for the treatment of mirror aneurysms.
The FDD was the first and unique treatment in 23 (96 %) patients; in one (4 %) the device was implanted in association with coils due to complex aneurysm morphology.
Two patients (8 %) harboring two contiguous aneurysms were treated by a single device (Table 1).
Aneurysm characteristics
Among the 24 aneurysms, ten (42 %) were located at the paraophthalmic internal carotid artery (ICA), five (21 %) at cavernous segment, eight (33 %) at the posterior communicating artery (PComA), and one (4 %) at one at anterior choroidal artery (AChoA).
All aneurysms were saccular. The aneurysm size ranged from 2 to 25 mm (median diameter, 8 mm). Twenty (83 %) were small, three (13 %) large, and one (4 %) giant.
The neck size ranged from 2 to 12 mm (average, 8 mm). The neck-sac ratio ranged from 0.5 to 0.8 (average, 0.6) (Table 1).
Endovascular treatment and medication
All patients were pre-treated with 75 mg daily of clopidogrel for 5 days, in association with 150 mg of aspirin. Thrombocyte inhibition tests were not performed routinely before the treatment. All patients had continuous intravenous infusion of heparin during the procedure, and a bolus of 1000 IU every hour during the procedure, to maintain an activated clotting time (ACT) >250–300 s.
The clopidogrel (75 mg) and aspirin (100 mg) were administered daily until the aneurysm occlusion was confirmed by DSA; then, only aspirin was continued indefinitely. Corticosteroids were not administered.
All endovascular treatments were performed by an interventional neuroradiologist with more than 20 years of experience of management of cerebral aneurysms (F.B).
The procedure was performed under general anesthesia through catheterism of the right common femoral artery by using a 8F vascular sheath and a triaxial system (Neuronmax Penumbra, LIR 5.5 Phenox, Headway 27 Microvention). The FRED size was chosen according to the proximal parent vessel diameter, as usual for the other FDD. The FRED was then placed at the level of the aneurysm. The correct apposition to the vessel wall was assessed with DSA and nonsubtracted angiographic images. The procedure was considered successful if the FRED completely covered the aneurysm.
Intra-aneurysmal contrast stasis was observed in all patients immediately at the end of the procedure.
Follow-up
The clinical-neurological evaluation was performed immediately after the procedure, at discharge and at 1 and 3-months.
Radiological examination by CTA was performed at 3, 6, and 12 months, until the aneurysm occlusion was obtained; in all cases, the complete aneurysm occlusion was then confirmed by DSA.
For patients with documented occlusion, a follow-up with contrast-enhanced magnetic resonance angiography (CE-MRA) was obtained every 12 months. The actual follow-up ranges between 6 and 24 months (mean 12 months).
Results
Treatment characteristics
All the patients had one device for each aneurysm; besides, in two patients one device was used for the treatment of two contiguous aneurysms.
No early or delayed aneurysm rupture, no subarachnoid or intraparenchymal hemorrhage (IPH), no ischemic complications, and no deaths occurred (mRS = 0).
There was one intra-procedural technical complication (4 %) in a patient harboring two mirror PComA aneurysms. One was successfully treated, whereas in the controlateral the deployment of the FRED failed because of a proximal calcified stenosis; thus, a balloon-assisted coiling was realized.
One patient developed a femoral pseudoaneurysm, resolved with compressive medication.
One patient experienced a severe episode of hemoptysis after the extubation, with consequent aspiration pneumonia, which was successfully treated with medical therapy.
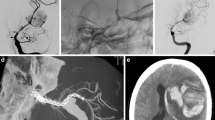
In another patient with a large PComA aneurysm, treated with coils and FDD, a severe carotid vasospasm occurred during coil deployment, which did not resolve after intra-arterial nimodipine (2 ml) administration and an angioplasty attempt. The patient had no ischemic complications due to the good collaterals and controlled hypertension. Restored flow and patency of the device with partial aneurysm occlusion was observed at 3 months and complete aneurysm occlusion was observed at 12 months (Fig. 1).
Radiological outcome
All patients underwent 3- and 6-month follow-up, 18 of them (90 %) were studied at 12 months and five (25 %) at 24 months.
Complete occlusion (OKM D) was obtained in 20 out of 24 aneurysms (83 %) and partial occlusion (OKM C) in four (17 %). Among the four partially occluded aneurysms (OKM C), two intracavernous had 6-months follow-up; the further outcome of these two aneurysms will be defined in a longer follow-up. Two other aneurysms were located at the PcomA and paraophthalmic ICA, respectively. The case of PcomA had a fetal origin of the ipsilateral posterior cerebral artery that could explain the incomplete occlusion at 1 year.
The complete occlusion (83 %) (20 patients) occurred at 3 months in ten cases (42 %), at 6 months in eight (33 %), and at 12 months in two (8 %) (Figs. 2, 3, and 4) (Table 2).
All FREDs were patent at the last follow-up. The correlation of the occlusion time with the aneurysm characteristics (Table 3) showed large prevalence of occlusion of small aneurysms at 3 months (50 %), as expected, and no difference of occlusion time according to the aneurysm size, neck, and neck–sac ratio.
Discussion
The aim of the treatment with FDDs is disrupting the intra-aneurysmal flow and to favor intra-aneurysmal thrombosis, while preserving patency of the adjacent small vessels. The FDD mesh porosity and the pressure gradient between the parent artery and the aneurysm sac play a significant role in the aneurysm occlusion time; in the same way, it ensures the patency of the side branches covered by the FDD.
Although it has not been proven that increasing the mesh density brings better results in terms of occlusion in vivo, modulation of the mesh porosity is a promising strategy; particularly it seems to improve the angiographic occlusion of experimental wide-necked aneurysms [15]. Computational tools for simulating clinical interventions and hemodynamics in patient-specific intracranial aneurysms helped to evaluate the impact of various intervention strategies and identify hemodynamic factors that affect treatment outcome [11, 32].
From a technical point of view, the FRED is a self-expanding nickel titanium, paired-stent, which integrates dual-layer coverage; it facilitates simultaneous deployment and partial resheathability. It has a dual-layer design at various target vessel diameters/lengths, 70 to 80 % of retrievability, good visualization under fluoroscopy due to tantalum proximal and distal total length markers and interweaved tantalum working length helical strands. It is tracked and deployed through a .027” (0.69 mm) ID microcatheter, like the Headway 27 (Microvention, Tustin, CA, USA) microcatheter. Its diameter ranges from 3.5 to 5.5 mm, so it is not recommended for small vessels (<3 mm).
The dual-layer structure of the FRED answers to the modulation of the stent porosity: a high-density mesh is designed to cover the aneurysm neck and occlude blood flow into the aneurysm while a low-density mesh is used to enable blood flow into adjacent small side branches, as perforators.
The use of FDD has been reported in the recent literature mainly to treat ICA aneurysms proximal to the circle of Willis while the experience with more distal aneurysms is very limited [3, 24]. Besides, mid- and long-term results are mainly available for the PED and less for the Silk FDD. Particularly, aneurysm occlusion rates, reported by a multicenter PED study [10] increased from 81.6, 84.1, to 93.2 % in the 6-month, 1-year, and 2-year time periods, respectively.
The experience with new FDD is very limited, particularly regarding the mid-term follow-up.
Four recent studies [12, 17, 20, 25] have reported the experience of treatment of cerebral aneurysms by FRED FDD. The data of these series and our own, including 103 patients with 107 aneurysms, are summarized in Table 4. The location was mainly the paraophthalmic ICA (71 aneurysms or 60 %), followed by PcomA location (16 or 14 %), cavernous segment (11 or 9 %), and posterior circulation (11 or 9 %), more rarely at AChoA (1 or 1 %), petrous/extracranial ICA (3 or 4 %) or anterior cerebral artery (ACA) (4 or 3 %).
The aneurysm morphology was mainly saccular (84 %), more rarely fusiform (13 %), and blister (3 %). The size was mainly small (86 %), more rarely large (8 %), and giant (6 %). Sixteen patients (14 %) had undergone a previous endovascular coiling, resulting in incomplete occlusion or recanalization.
FDD was the unique treatment in 88 %; additional coiling was performed in 13 patients (9 %), while in one (1 %) FDD was implanted in association of a traditional stent.
The rate of complete occlusion, reported only in four studies [17, 20, 25] (including ours), was 45/78 (58 %) at 3 months, 44/59 (74 %) at 6 months, and 28/30 (93 %) at 12 months. No deaths occurred in the analyzed series. Complications were classified into technical intra-procedural and post-procedural.
Intraprocedural technical complications occurred in five patients (5 %) including failure to device deployment in four [12] and a dissection of the petrous ICA in one [17]. No permanent morbidity related to these complications was reported in the analyzed series.
Only ten out of 117 patients (8 %) had post-procedural complications, both early and delayed; there were two minor strokes and one major stroke [16], two transient ischemic attacks [15], two asymptomatic in stent-thrombosis [16, 19], a femoral pseudoaneurysm, a severe vasospasm, an aspiration pneumonia. Permanent morbidity was reported in two patients (2 %) [20, 25].
Analyzing the data available in the literature (Table 4), the FRED has particularly been used in the treatment of ICA siphon (84 %) and small aneurysms (86 %).
The good results reported in our analysis are probably related to the very low rates of large (8 %) and giant (6 %) aneurysms, and those located over the circle of Willis (4 %) or in the posterior circulation (PC) (9 %). In the group of large aneurysms whose follow-up was available, only 6/8 (75 %) [20, 25] were occluded at the last follow-up, while the occlusion rate of giant aneurysms was even lower (57 % or 4/7). Conversely, aneurysms located beyond the circle of Willis, as ACA [17, 20], showed complete occlusion at the last follow-up, at 3, 6, and 12 months, respectively.
Aneurysms of the PC [12, 17, 20], treated with FRED, showed late occlusion; in fact, among the 9/11 patients whose follow-up is reported, the angiographic occlusion at the last follow-up occurred in 5/9 aneurysms (55 %). As reported in a recent meta-analysis, patients harboring PC aneurysms treated by FDD are at significantly higher risk of mortality, ischemic stroke, and perforator infarctions [30]. Thus, in the PC, the occlusion with the FRED should probably be delayed; besides, the lack of mortality in all the analyzed series is promising.
The rates of complete occlusion at the last follow-up, morbidity and mortality in different series using other FDD, as SILK, PED, SURPASS, and p64, are summarized in Table 5. Particularly for the SILK studies, the morbidity ranges from 5 to 8.7 %, the mortality from 0 to 5 %, and the occlusion rate from 59 to 86 %. On the other hand, for the PED, the morbidity ranges from 1 to 5.6 %, the mortality from 0.5 to 6 % and the occlusion rate from 52 to 94.6 %.
The experience with newer devices, such as SURPASS and p64 is initial. A recent study with SURPASS [29] reports 4.2 % morbidity, 2.4 % mortality, and 75 % occlusion rate while p64 [13] shows 1.7 % morbidity, 0.8 % mortality, and 85.7 % occlusion rate.
When compared with the PED, the FRED shows similar or even better profile, with high occlusion rate (93 %), no mortality, and low permanent morbidity (2 %).
Limitations of our study are its retrospective nature, the inclusion of a limited number of patients, the mid-term follow-up, the main aneurysm location in the paraophthalmic ICA (42 %), and the large prevalence of small aneurysms (83 %). On the other hand, FDD was the first and unique treatment in all our patients, except for one aneurysm treated with additional coiling; besides the follow-up, using noninvasive imaging (CTA and CE-MRA) in addition to DSA was available in all cases; finally, our study includes the largest number of patients with a 12-month angiographic follow-up.
Conclusions
The endovascular treatment with FRED FDD is a safe treatment for unruptured cerebral aneurysms, resulting in a high rate of occlusion.
The FRED has resulted substantially equivalent to the others known FDDs which show similar functions and technical profiles.
Nevertheless, further series with a larger patient population and long-term follow-up will define its role in the treatment of intracranial aneurysms.
References
Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M (2013) Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery 73:193–9, discussion 199–200
Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, Kadziolka K, Barreau X, Dousset V, Bonafé A (2012) Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 33:1150–1155
Briganti F, Delehaye L, Leone G, Sicignano C, Buono G, Marseglia M, Caranci F, Tortora F, Maiuri F (2016) Flow diverter device for the treatment of small middle cerebral artery aneurysms. J Neurointerv Surg 8:287–94
Briganti F, Leone G, Marseglia M, Cicala D, Caranci F, Maiuri F (2016) p64 flow modulation device in the treatment of intracranial aneurysms: initial experience and technical aspects. J Neurointerv Surg 8:173–80
Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, Maiuri F (2015) Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 28:365–75
Briganti F, Napoli M, Leone G, Marseglia M, Mariniello G, Caranci F, Tortora F, Maiuri F (2014) Treatment of intracranial aneurysms by flow diverter devices: long-term results from a single center. Eur J Radiol 83:1683–90
Briganti F, Napoli M, Tortora F, Solari D, Bergui M, Boccardi E, Cagliari E, Castellan L, Causin F, Ciceri E, Cirillo L, De Blasi R, Delehaye L, Di Paola F, Fontana A, Gasparotti R, Guidetti G, Divenuto I, Iannucci G, Isalberti M, Leonardi M, Lupo F, Mangiafico S, Manto A, Menozzi R, Muto M, Nuzzi NP, Papa R, Petralia B, Piano M, Resta M, Padolecchia R, Saletti A, Sirabella G, Bolgè LP (2012) Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications—a retrospective data analysis. Neuroradiology 54:1145–1152
Brinjikji W, Mohammad HM, Lanzino G, Cloft HJ, Kallmes DF (2013) Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 44:442–7
Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M (2010) Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One 5:e12492
Chiu AH, Cheung AK, Wenderoth JD, De Villiers L, Rice H, Phatouros CC, Singh TP, Phillips TJ, McAuliffe W (2015) Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 1728–34
Damiano RJ, Ma D, Xiang J, Siddiqui AH, Snyder KV, Meng H (2015) Finite element modeling of endovascular coiling and flow diversion enables hemodynamic prediction of complex treatment strategies for intracranial aneurysm. J Biomech 48:3332–40
Diaz O, Gist TL, Manjarez G, Orozco F, Almeida R (2014) Treatment of 14 intracranial aneurysms with the FRED system. J Neurointerv Surg 6:614–7
Fischer S, Aguilar-Pérez M, Henkes E, Kurre W, Ganslandt O, Bäzner H, Henkes H (2015) Initial experience with p64: a novel mechanically detachable flow diverter for the treatment of intracranial saccular sidewall aneurysms. AJNR Am J Neuroradiol 36:2082–9
Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H, Henkes H (2012) Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 54:369–382
Gentric JC, Salazkin I, Gevry G, Raymond J, Darsaut T (2015) Compaction of flow diverters improves occlusion of experimental wide-necked aneurysms. Neurointerv Surg, pii: neurintsurg-2015-012016
Kallmes D, Ding YH, Daying D, Kadirvel R, Lewis DA, Cloft HJ (2007) A new endoluminal, flow-disrupting device for the treatment of saccular aneurysms. Stroke 38:2346–52
Kocer N, Islak C, Kizilkilic O, Kocak B, Saglam M, Tureci E (2014) Flow re-direction endoluminal device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 120:1158–71
Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F (2015) Silk flow-diverter stent for the treatment of intracranial aneurysms: a series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol 36:542–6
Maimon S, Gonen L, Nossek E, Strauss I, Levite R, Ram Z (2012) Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien) 154:979–87
Möhlenbruch MA, Herweh C, Jestaedt L, Stampfl S, Schönenberger S, Ringleb PA, Bendszus M, Pham M (2015) The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 36:1155–61
Mpotsaris A, Skalej M, Beuing O, Eckert B, Behme D, Weber W (2015) Long-term occlusion results with SILK flow diversion in 28 aneurysms: do recanalizations occur during follow-up? Interv Neuroradiol 21:300–10
O’Kelly CJ, Krings T, Fiorella D, Marotta TR (2010) A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 16:133–7
O’Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A, Willinsky RA, Kelly M, Marotta TR (2013) Canadian experience with the pipeline embolization devices for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 34:381–7
Pistocchi S, Blanc R, Bartolini B, Piotin M (2012) Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke 43:1032–8
Poncyljusz W, Sagan L, Safranow K, Rac M (2013) Initial experience with implantation of novel dual layer flow-diverter device FRED. Wideochir Inne Technol Maloinwazyjne 8:258–64
Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS (2012) Treatment of intracranial aneurysms using the Pipeline flow-diverter device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 33:1436–1446
Shankar JJ, Tampieri D, Iancu D, Cortes M, Agid R, Krings T, Wong J, Lavoie P, Ghostine J, Shettar B, Ritchie K, Weill A (2016) SILK flow diverter for complex intracranial aneurysms: a Canadian registry. J Neurointerv Surg 8:273–8
Wagner A, Cortsen M, Hauerberg J, Romner B, Wagner MP (2012) Treatment of intracranial aneurysms. Reconstruction of the parent artery with flow-diverting (Silk) stent. Neuroradiology 54:709–718
Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A, Hartmann M, Szikora I, Pierot L, Sakai N, Imamura H, Sourour N, Rennie I, Skalej M, Beuing O, Bonafé A, Mery F, Turjman F, Brouwer P, Boccardi E, Valvassori L, Derakhshani S, Litzenberg MW, Gounis MJ, Surpass Study Group (2015) Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol 36:98–107
Wang CB, Shi WW, Zhang GX, Lu HC, Ma J (2016) Flow diverter treatment of posterior circulation aneurysms. A meta-analysis. Neuroradiology 58:391–400
Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, Leung KM, Lee R, Cheng HK, Cheung YL, Chan CM, Wong GK, Hui JW, Wong YC, Tan CB, Poon WL, Pang KY, Wong AK, Fung KH (2012) Intracranial aneurysms: mid-term outcome of pipeline embolization devices—a prospective study in 143 patients with 178 aneurysms. Radiology 265:893–901
Zhang Y, Chong W, Qian Y (2013) Investigation of intracranial aneurysm hemodynamics following flow diverter stent treatment. Med Eng Phys 35:608–15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Conflict of interest
F.B. serves as proctor for COVIDIEN with a modest remuneration.
The other authors have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Briganti, F., Leone, G., Ugga, L. et al. Safety and efficacy of flow re-direction endoluminal device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir 158, 1745–1755 (2016). https://doi.org/10.1007/s00701-016-2875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2875-4