Article Text
Abstract
Introduction This report details experience with the Neuroform stent, with an emphasis on evolving treatment strategies, complication rates and treatment durability.
Methods All patients undergoing Neuroform stent assisted aneurysm treatment were registered in prospectively maintained endovascular databases at two institutions.
Results 284 patients with 302 aneurysms underwent aneurysm treatment with Neuroform during a 42-month study period. Imaging follow-up was available for 166 of 286 saccular aneurysms which were treated with stents and coils (average interval 12.9 months). 80 demonstrated progressive thrombosis (48.2%), 40 were unchanged (24.1%) and 46 (27.7%) demonstrated re-canalization, 25 (15.1%) of which were major recanalizations requiring retreatment. The vast majority of recanalizations and retreatments were observed in large or giant aneurysms. A cumulative total of 25 ischemic strokes (8.8%) and eight neurovascular deaths (2.8%) were recorded in these patients. Ten of these strokes were associated with transient deficits which went on to complete resolution by the time of discharge or at the initial clinical follow-up, yielding a significant stroke rate of 5.3%. Delayed (>48 h) complications, including four deaths—related to stroke (n=2, 6 days and 8 weeks post-procedure) and spontaneous parenchymal hemorrhages (n=2)—represent events which are a direct consequence of stenting and likely would not have been encountered in the context of standard non-stent supported embolization techniques.
Conclusion Neuroform facilitates the endovascular treatment of complex and wide necked cerebral aneurysms. However, complete occlusion at angiographic follow-up remains uncommon and is observed in only one-third of patients. Delayed complications (>48 h) represent an important component of the overall complications associated with Neuroform assisted aneurysm embolization.
- Artery
- Aneurysm
- Stent
Statistics from Altmetric.com
The initial experience with the Neuroform stent has indicated that the device can be deployed safely within the cerebral vasculature, facilitating the treatment of complex cerebral aneurysms, many of which otherwise may not have been amenable to traditional endovascular treatment strategies.1–3 Evolving data suggest that the Neuroform may also augment the durability of aneurysm treatment.4 We present our two institution experience with the Neuroform stent, with an emphasis on evolving treatment strategies, treatment durability at long term follow-up and complication rates. These efficacy and durability data, assessed within the context of the morbidity and mortality associated with endovascular treatment, can ultimately function to help guide an evidence based approximation of the risk:benefit ratio associated with the endovascular treatment of complex cerebral aneurysms in individual patients.
Methods
Patients
All patients undergoing aneurysm treatment with the Neuroform stent at our two institutions were registered into prospectively maintained endovascular databases. We reviewed these databases and retrospectively adjudicated and supplemented the recorded data using the inpatient and outpatient clinical charts, dictated operative reports and all available imaging studies. IRB approval for this study was obtained at both participating institutions.
Stenting technique
Delivery and deployment of the Neuroform stent was performed as previously described.1 4 In brief, all procedures were performed under general anesthesia. All patients were fully anticoagulated (activated clotting time > 200 s) for the procedure. A variety of platelet inhibition strategies were employed over the course of the series. The majority of patients were pretreated with aspirin and clopidogrel, typically for at least 4 days. If patients were not pretreated with oral antiplatelet agents, abciximab was administered (either intravenously or intra-arterially) just prior to, or immediately after, stent deployment. Embolization coils used included Matrix Detachable Coils (Boston Scientific, Natick, Massachusetts, USA), Guglielmi Detachable Coils (GDC, Boston Scientific), HydroCoil Embolic System (Microvention Inc, Aliso Viejo, California, USA), Sapphire, NXT and Nexus Coils (EV3 Neurovascular, Irvine, California, USA) and Micrus ACT, Cerecyte and Presidio MicroCoil Systems (Micrus, Sunnyvale, California, USA). Following the procedure, heparinization was not reversed. In most cases, an arterial closure device (Angioseal, St Jude Medical, St Paul, Minnesota, USA; Perclose, Abbott Vascular Devices Redwood City, California, USA; Starclose, Abbott Vascular Devices) was used to achieve hemostasis at the puncture site. Patients were continued on both aspirin and clopidogrel for between 4 and 6 weeks with aspirin therapy continued indefinitely thereafter.
Imaging follow-up
Follow-up conventional angiography was performed at high magnification in the original working angle for coil embolization, optimized by replicating the relationship of osseous landmarks to the coil mass and the Neuroform stent(s). Follow-up MR angiography (MRA) examinations were compared with the initial post-treatment MRA (typically performed between 6 and 72 h after the initial treatment) as well as the immediate post-embolization angiogram. Any increased aneurysm filling was termed “re-canalization”. Any decreased filling was termed “progressive thrombosis”. No perceptible change between studies was termed “no change”. All aneurysm re-canalizations requiring retreatment were also documented. All imaging examinations were evaluated by one neuroradiologist (DF).
Clinical follow-up and adjudication of complications
Inpatient and outpatient clinic charts and all available post-procedural imaging examinations were reviewed for evidence of complications. Complications were categorized as periprocedural if they occurred during or within 48 h of the procedure and delayed if they were identified later during the hospital course or after discharge. Ischemic strokes were assigned as any new neurological deficits which either persisted beyond 36 h or were transient, but associated with new lesions on diffusion imaging (ie, a “clinical transient ischemic attack with positive diffusion imaging”). Ischemic strokes were considered minor if the deficit had resolved completely by the time of discharge or the initial clinical follow-up.
Results
Patient population
A total of 284 patients with 302 aneurysms were treated with Neuroform stent support or with the Neuroform stent alone between November 2002 and May 2006 (42 months). Two hundred and eighty-six were saccular aneurysms treated with stents and coils and 16 were fusiform aneurysms which were treated with stents alone. Sixteen patients had multiple aneurysms which were treated with one or more stents during the same or different settings. Five patients had bilateral aneurysms treated with Neuroform stent support in different vascular distributions. The anatomical location of the treated lesions is listed in table 1. The majority of aneurysms treated were either unruptured or had a remote (>6 month) history of subarachnoid hemorrhage with the aneurysm initially secured with either clipping or coiling with subsequent recurrence treated with stent support. Only 29 patients were treated prospectively with the stent in the context of acute (<4 weeks) or subacute (<6 months) subarachnoid hemorrhage. In 21 cases, the stent was used during the primary treatment of a ruptured aneurysm. In eight cases, treatment was staged with a partial coiling performed initially to secure the aneurysm followed by stent assisted coiling during a second stage.
Saccular aneurysm location
Evolution of stenting technique
Initially, stenting was performed as an adjunct to coil embolization with the stent placed prior to the introduction of embolization coils. Later in the experience, embolization was sometimes performed initially with a balloon assisted technique followed by placement of the stent after all or most of the aneurysm had been embolized with coils. In addition, more complex approaches to parent vessel reconstruction were developed during the course of the series.
For complex terminus aneurysms, a Y stenting technique has been applied in which one limb of the bifurcation is stented followed by the placement of a second stent through the initial stent into the second limb of the bifurcation (figure 1).
A patient with an incidental basilar artery apex aneurysm. Non-invasive and conventional angiographic imaging demonstrates a wide necked basilar apex aneurysm incorporating both proximal P1 segments of the posterior cerebral arteries (PCAs) (A). Unsubtracted frontal image (B) following treatment demonstrates Neuroform stents in place extending from the distal basilar into the right and left PCAs, respectively. Two sets of four proximal markers (arrows) are demonstrated within the distal basilar with single sets of four radio-opaque markers within each PCA (arrows). Final unsubtracted control angiography demonstrates the stent construct and coil mass in relation to the anatomy of the contrast opacified vasculature (C).
The application of a single stent across the neck of a terminal aneurysm represents a second approach to achieve reconstruction and was applied successfully to treat three of the aneurysms in the present series.
Imaging follow-up
Imaging follow-up is available for 166 saccular aneurysms and 11 non-saccular aneurysms. Of the 166 saccular aneurysms with imaging follow-up, the most recent follow-up examination was performed with conventional angiography in 125 cases and short TE MRA in 41. Of the 41 patients with MRA follow-up, 21 had at least one conventional angiogram for follow-up after the initial treatment. All patients with MRA follow-up also had a baseline MRA performed at the time of the last conventional angiographic examination which could be used for direct comparison to the follow-up examination.
Saccular aneurysms were subdivided into anatomical categories using the UCLA classification scheme5 with small aneurysms (<10 mm) divided into small (<4 mm; SASN) and wide necked (>4 mm; SAWN) categories, large aneurysms (10–25 mm) and giant aneurysms (>25 mm). In the population of aneurysms with follow-up, 25 were SASN, 72 were SAWN, 57 were large and 12 were giant. The average follow-up interval for the group of saccular aneurysms was 12.9 months (range 2–37 months). Eighty demonstrated progressive thrombosis (48.2%), 40 were unchanged (24.1%) and 46 (27.7%) demonstrated recanalization, 25 (15.1%) of which were major re-canalizations requiring re-treatment (table 2). The vast majority of recanalizations and retreatments were observed in large or giant aneurysms. Small aneurysms re-canalized at a rate of 9.3% (9/97) with only three of 97 (3.1%) lesions demonstrating major re-canalization requiring retreatment. Of those lesions with follow-up, 60.8% were either completely occluded (33.1%) or nearly completely occluded (27.7%) with small residual neck remnants at the aneurysm–parent vessel interface at the latest follow-up examination.
Neurological complications
Of the 15 non-saccular aneurysms treated, 10 were “intradural pseudoaneurysms” (ie, blood blister-like aneurysms, dissecting aneurysms and aneurysms arising at the site of prior clipping of a ruptured aneurysm but not representative of a recurrent or residual of the original aneurysm neck; PSAs), seven of which were treated in the setting of acute or subacute subarachnoid hemorrhage (SAH) and three of which had a remote history of SAH. Five were fusiform aneurysms, which were unruptured. All 10 patients with intradural PSAs have undergone angiographic follow-up (average 9 months, range 1–18.5%) demonstrating complete (n=5) or near complete (n=4) resolution in nine patients and stability in one.6
Complications
Any complications incurred prior to stenting (ie, patients with aneurysms which were previously coiled or clipped) were not included. For the purpose of calculating the per cent incidence of each type of complication, the total number of patients (n=284) treated was used as the denominator—irrespective of the number of stages required to treat the lesion(s) (including retreatments of recurrent aneurysms), the number of lesions treated and the number of vascular distributions in which those lesions were treated.
Procedural and periprocedural (<48 h) complications
Early complications were divided into neurological and access site events (table 2). Nineteen periprocedural strokes occurred (6.7%). Of these patients, eight had minimal deficits at presentation which resolved completely prior to discharge or at the time of the initial clinical follow-up. There were five deaths (1.8%), all of which were associated with new infarctions (two were also associated with concurrent SAH). Five procedural perforations resulted in a new SAH. In two cases, no extravasation of contrast was noted during the procedure, with extravascular contrast identified only on post-procedural imaging. Three of these events were well tolerated, and two were associated with a concurrent major stroke and death. Two patients (0.7%) experienced transient neurological deficits which completely resolved within 48 h of the procedure and were not associated with any abnormality on MR imaging with diffusion.
There were 26 access site complications, six (2.1%) of which resulted in hemodynamic instability or required surgical intervention.
Delayed complications (>48 h)
Following aneurysm treatment, eight patients (2.8%) experienced strokes (table 2). Two could be attributed to delayed in-stent stenosis. Two patients with delayed strokes had subacute stent thrombosis and subsequently died. Of these strokes, symptoms were minor and completely resolved in two cases. An additional four patients presented (1.4%) with parenchymal hematomas, two of whom died. Three were spontaneous (occurring 7 days, 6 weeks and 6 weeks after the procedure) and one was iatrogenic (occurring on post-procedure day 14) related to placement of an external ventricular drain while the patient was on dual antiplatelet therapy. In the three patients with spontaneous hemorrhages, two were receiving dual antiplatelet medication and one was receiving both warfarin and aspirin. In total, there were four delayed deaths which could be attributed to vascular neurological events (1.4%).
Seven patients experienced delayed transient ischemic attacks (TIAs) which resolved with renewed compliance to, or resumption of, dual antiplatelet medications. Two patients presented with neurological symptoms which could be attributed to progressive mass effect secondary to aneurysm recanalization (one hemisensory deficit, one sixth nerve palsy). Two transient cases of perianeurysmal inflammation were noted with adjacent parenchymal T2 signal change and contrast enhancement which resolved with steroid therapy. One patient developed hydrocephalus after aneurysm embolization.
Delayed in-stent stenosis was identified in 10 of 177 patients (5.6%) with follow-up. Three patients were symptomatic, two with stroke and one with TIA. One of the strokes resulted in left hemiparesis. One resulted in transient (resolved in <24 h) left hemisensory symptoms but was associated with small infarctions on diffusion imaging. The patient with TIAs only experienced resolution of symptoms after the re-initiation of dual antiplatelet medications. The remaining patients were asymptomatic. Of the six asymptomatic patients with angiographic follow-up to date, partial or complete resolution of the stenosis has been observed in five.7
No aneurysmal SAH was observed during the follow-up period.
Overall major complications
A total of 25 ischemic strokes (8.8%) and eight neurovascular deaths (2.8%) occurred during the course of treatment and during the post-procedural period. Of the strokes, 10 were clinical TIAs with small foci of signal abnormality on diffusion imaging or were associated with minimal deficits at presentation which went on to complete resolution before discharge or at initial clinical follow-up, yielding a significant stroke rate of 5.3%.
Discussion
A number of single institution case series have demonstrated that the Neuroform stent facilitates the endovascular treatment of complex and wide necked cerebral aneurysms.3 6 8–12 Since the introduction of Neuroform, innovative applications have evolved which have been applied to achieve increasingly complex endovascular reconstructions and further broaden the spectrum of aneurysms amenable to endovascular therapy.13–16 Emerging data suggest that in addition to functioning as an adjunctive device to support the coil embolization of aneurysms, Neuroform may augment the durability of aneurysm embolization through a combination of hemodynamic and biological effects.17–19 The current review of our prospectively maintained database was performed to assess the durability of Neuroform assisted embolization of complex aneurysms at long term follow-up and to evaluate the safety profile of treating such lesions with an endovascular strategy that employs the stent. These efficacy and durability data, assessed within the context of the morbidity and mortality associated with endovascular treatment, can ultimately function to help guide an evidence based approximation of the risk:benefit ratio associated with the stent supported endovascular treatment of complex cerebral aneurysms in individual patients.
Durability of aneurysm embolization
As assessment of the recanalization rates of the aneurysms included in the current series is somewhat complicated by the lack of any analogous control group. The existing longitudinal series assessing the durability of aneurysm embolization include aneurysms which were exclusively or nearly exclusively treated without the use of adjunctive devices. Correspondingly, the anatomy of these lesions is completely different from those included in the current study, most of which could not be treated without an adjunctive device and many of which could not have been treated in the absence of the durable parent vessel protection provided by an intravascular stent. The most analogous control group would be a population of aneurysms treated exclusively with a balloon remodeling technique but unfortunately no large study of this type currently exists. Even given this limitation, the current data compare favorably with the previously reported rates of aneurysm re-canalization after coil embolization.20 In the current series, the overall recanalization rate of 27.7% compares favorably with the 33.6% recanalization rate reported by Raymond and colleagues20 in an unselected group of aneurysms with a similar follow-up interval. If the giant aneurysms in the present series are removed from consideration, the overall re-canalization and retreatment rates improve substantially to 22.7% and 11.7%. The results for the SAWN category are especially compelling, with re-canalization and retreatment rates of 8.3% and 2.8%, respectively. The 8.3% re-canalization rate for these aneurysms is considerably lower than the 20% re-canalization rate observed by Murayama for aneurysms with these dimensions.5
While the durability results for aneurysms less than 10 mm were generally excellent, the complex large and giant aneurysms in the present study continued to demonstrate very high rates of recanalization (53.6%). These aneurysms remain challenging and at the outset the operator should expect that many of these lesions will demonstrate recurrence and likely require additional stages of embolization to achieve an adequate level of long term occlusion.
Rates of progressive thrombosis
In the absence of the application of Neuroform, the observation of progressive thrombosis on follow-up angiography is not common, seen in approximately 25% of cases.5 In the current series, progressive thrombosis was the most common finding on follow-up angiography, observed in 48.2% of all aneurysms and 60.8% of small aneurysms. It is likely that this observation, at least in part, represents an epiphenomenon related to the application of heparin, aspirin and clopidogrel in these patients at the time of the initial treatment. While the discontinuation of anticoagulation and dual antiplatelet therapy is very likely to account for the resolution of small foci of residual filling within the interstices of the coil mass, the resolution of neck remnants and aneurysm residuals adjacent to the parent vessel–aneurysm interface suggests a concomitant component of endovascular remodeling (figure 2) as it is very unusual to see these types of residuals resolve at follow-up under other circumstances.
A patient with bilateral wide necked internal carotid artery (ICA), carotid–ophthalmic segment aneurysms, both of which were electively treated with stent supported embolization. The pretreatment angiogram (A) demonstrates the ICA in a “down-the-barrel” orientation with the aneurysm neck incorporating nearly 180° of the circumference of the parent vessel. Following embolization, there is residual filling in the region of the aneurysm neck medially (arrow) and into the aneurysm fundus through the interstices of the coil mass (B). Follow-up angiography performed at 11 months (C) in the working angle for embolization demonstrates complete aneurysm occlusion with resolution of all residual filling, including those regions at the aneurysm–parent vessel interface.
Rates of complete occlusion
In the present series, the majority of aneurysms treated were not completely occluded at angiographic follow-up — only one-third of the lesions treated were angiographically cured. While Neuroform facilitates, and in some cases actually makes feasible, the embolization of these more complex lesions, the treatments are often not “curative”. At the same time, the significance of the small neck remnants which were left in some cases remains largely unknown.
Efficacy of stent monotherapy and in-stent stenosis: further evidence of “endovascular remodeling”
Hypothetically, endovascular stents may improve the durability of aneurysm treatment by modifying the flow dynamics and also inducing “biological remodeling” at the aneurysm–parent vessel interface.
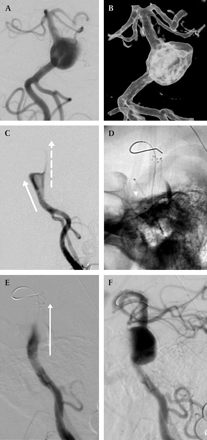
An elegant set of in vitro experiments performed by Canton and colleagues17 18 have demonstrated that Neuroform, despite its minimal metal surface area coverage, can significantly alter the baseline hemodynamics in experimental aneurysms by reducing the velocity of the inflow jet and reducing intra-aneurysmal vorticity and shear stress. In some cases, these changes are evident clinically as substantial changes in the dynamics of contrast flow following endovascular reconstruction with Neuroform (figure 3).
A patient with a circumferential basilar trunk aneurysm which was treated using a “balloon in-stent” technique. Initial conventional (A) and three-dimensional (B) angiographic views demonstrate a circumferential aneurysm incorporating 360°of the mid basilar trunk, projecting eccentrically to the left. The first frame from the lateral angiogram demonstrating arterial contrast depicts two distinct flow jets (C). The dominant flow jet (solid arrow) projects anteriorly into the aneurysm dome. In addition, a less robust column of flow (broken arrow) projects directly superiorly, along the trajectory of the basilar artery. Unsubtracted lateral image (D) following the placement of two Neuroform stents within the native basilar artery demonstrates a microcatheter (arrowhead) projecting into the aneurysm along the trajectory of the dominant flow jet while the microwire passes through the stent to demarcate the anatomical course of the parent basilar artery. The first frame from the lateral angiogram (E) depicting arterial contrast performed after the placement of two Neuroform stents demonstrates the dominant flow jet (arrow) now directed along the trajectory of the normal basilar artery. The anterior flow jet into the aneurysm is no longer visualized. A later frame from the lateral angiogram (F) depicts contrast within the basilar apex and slowly transiting into the aneurysm through the interstices of the stents to fill the superior aspect of the aneurysm.
Lopes and Sani19 presented findings from a single patient who underwent autopsy 4 months following Neuroform stent placement. Although no embolization coils could be introduced into the aneurysm at the time of the initial treatment, histopathological evaluation of the parent vessel–aneurysm complex demonstrated intimal thickening within the parent vessel over the stented segment with de novo fibroelastic tissue growing along the edges of the aneurysm neck. This provocative observation supporting the concept that the stent may stimulate some level of proliferative “bioresponse” at the parent vessel–aneurysm interface is concordant with our observations of pseudoaneurysm resolution after stent monotherapy (figure 4) and the development of angiographically moderate to severe in-stent stenosis in some patients (figure 5). In the case of pseudoaneurysm treatment, we hypothesize that the flow redirection, in combination with progressive stent endothelialization and some component of intimal remodeling or hyperplasia, results in endoluminal remodeling which results in gradual healing of the aneurysmal segment of the vascular wall. In some cases, this “endoluminal remodeling” is particularly robust, with the proliferative response to the stent subsequently culminating in in-stent stenosis. This extreme end of the endovascular remodeling spectrum is relatively uncommon (occurring in approximately 5% of patients), is usually asymptomatic and often resolves (partially or completely) with time. While the induction of in-stent stenosis over a previously angiographically normal vascular segment is clearly an undesirable consequence of stent supported aneurysm treatment, this observation, at the same time, suggests that the majority of patients likely demonstrate less vigorous degrees of remodeling which may augment treatment durability.
A patient with subarachnoid hemorrhage. CT of the brain (A) demonstrated diffuse perimesencephalic and posterior fossa subarachnoid hemorrhage with a large focal clot distributed circumferentially about the distal basilar trunk. An initial conventional angiogram was interpreted as normal. In retrospect, an oblique projection following the left vertebral injection (B) demonstrates a tiny (<1 mm) bleb (arrow) projecting along the left lateral surface of the mid-basilar artery. Follow-up angiography (C) in the same oblique projection performed at 6 weeks demonstrates the bleb (arrow) to have considerably enlarged in size, now measuring slightly greater than 2 mm. This “intradural pseudoaneurysm”, arising at the origin of a large perforator, was not amenable to surgical clipping or wrapping. The aneurysm neck was too small to accommodate a microcatheter, and the sac was too small to safely accommodate an embolization coil. For this reason, the lesion was treated with stent monotherapy. An unsubtracted image (D) demonstrates two Neuroform stents in place across the lesion. Axial source image from a short TE MR angiogram with contrast (E) performed immediately after stenting demonstrates the aneurysm arising from the left lateral aspect of the mid-basilar and projecting posteriorly (arrowhead). MRA at 8 weeks (F) demonstrates healing of the lesion (arrow). Follow-up conventional angiography in the oblique projection (G) demonstrates complete resolution of the lesion.
A patient with a large wide necked unruptured aneurysm of the posterior wall of the internal carotid artery. Conventional angiography (A) following the initial stent supported embolization demonstrates residual filling along the entire aneurysm neck and into the aneurysm fundus inferiorly (arrow). An unsubtracted image (B) demonstrates the Neuroform stent in position bridging the coil mass. Follow-up angiography at 9.5 months (C) demonstrates moderate (60–70%) diffuse in-stent stenosis along the entire mid portion of the stent. The aneurysm has progressed to complete occlusion with total obliteration of contrast filling in the region of the aneurysm neck. The patient was symptomatic with transient ischemic attacks which resolved immediately after reinstitution of dual antiplatelet therapy.
Complications
Several of the patients included in the current series had multiple aneurysms treated during the same session. In addition, many of the more complex aneurysms were treated over several stages or required retreatment due to recanalization. All complications encountered during all phases of treatment, including retreatments for recanalization, were taken into consideration. Because the complications were calculated using the number of patients (rather than the number of procedures or number of aneurysms treated) as the denominator, the reported rates represent the most conservative view of the present data.
Complications related to the non-stent supported embolization of unruptured aneurysms are typically observed within the immediate periprocedural period (<48 h).21 The periprocedural complication rate for the present series of complex aneurysms treated with stent support was comparable with those reported by other investigators for non-stent supported aneurysm embolization.22 Predictably, the majority of complications encountered were thromboembolic, with 19 periprocedural strokes (6.7%), many of which were minor at presentation and ultimately not associated with any permanent neurological deficit or disability (3.9% significant stroke rate).
When aneurysm treatment is performed in conjunction with the application of a stent, several factors contribute to an additional set of delayed complications. Firstly, the amount of potentially thrombogenic material within the parent vessel is greater than with non-stent supported endosaccular aneurysm treatment, increasing the possibility of delayed thromboembolic events. Secondly, for the control of thromboembolic events, these patients require long term treatment with dual antiplatelet agents, introducing the possibility of delayed hemorrhagic complications. Finally, the possibility of symptomatic in-stent stenosis also presents the potential for ischemic complications months after treatment. These delayed complications observed more than 48 h after stent assisted aneurysm embolization would not have been expected after standard non-stent supported embolization.
Delayed in-stent stenosis did not represent a significant component of the permanent morbidity or mortality rates observed in the current series.
Limitations
The present series represents a retrospective review of prospectively maintained clinical databases from two institutions. The results of the procedures were adjudicated by a single operator (DF) who participated in the majority of cases. This study design introduces several important limitations. Firstly, as with many of the available “real world” endovascular neurosurgical series, a significant proportion of the patients (nearly half) were lost to follow-up, or did not get follow-up, during the study period. Secondly, the clinical and imaging results are “self-adjudicated”, introducing the potential for bias. Despite these limitations, this study represents the largest reported series of patients treated with the Neuroform stent who have had mid-term to long term imaging and clinical follow-up.
Summary
The Neuroform stent facilitates the treatment of complex aneurysms. Evolving techniques continue to increase the complexity of endovascular reconstruction, which can be achieved and further increase the spectrum of aneurysms amenable to endovascular therapy. Stent use may augment the durability of aneurysm embolization. This durability advantage is most evident for small aneurysms with wide necks, which demonstrated low rates of recanalization and rarely required retreatment in the present series. We hypothesize that this durability advantage may be related to a combination of both the hemodynamic and biological effects (ie, “endovascular remodeling”) achieved with stenting. The efficacy of stent monotherapy for the treatment of pseudoaneurysms and the observation of delayed in-stent stenosis in some patients provides additional support for these concepts. The periprocedural complications associated with stent use are within range for those observed in large series of aneurysm embolization. Delayed complications, both ischemic (resulting from the thrombogenicity of the stent) and hemorrhagic (as a sequela of antiplatelet therapy), represent an important component of the overall rates of morbidity and mortality associated with Neuroform use. In-stent stenosis was not common, rarely symptomatic and associated with significant complications in only one patient. While Neuroform facilitated the endovascular treatment of these complex lesions, it is important to acknowledge that complete aneurysm occlusion was only observed in approximately one-third of cases.
References
Footnotes
Competing interests DF (Boston Scientific, research support, consulting fees, honoraria), PR and CM (Boston Scientific, consulting fees, honoraria and proctoring fees), FCA (Boston Scientific, proctoring fees). The Cleveland Clinic (PI DF) received a research grant from Boston Scientific in 2005 which partially supported this work.
Ethics approval Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.