Article Text
Abstract
Background Due to anatomic features, including wide necks and incorporation of important branches, endovascular coiling of middle cerebral artery (MCA) aneurysms has proved challenging. Stent assisted embolization may increase the likelihood of successful treatment.
Methods Consecutive patients undergoing stent assisted coil embolization utilizing the Neuroform stent from 2004 to 2009 were identified by hospital billing records. Procedural and clinical information—including procedure related mortality and morbidity and long term outcomes—were then obtained by retrospective chart review.
Results Treatment was successful in 22/23 (96%) patients. Median age was 61 years and 16/22 (73%) were women. Aneurysm size was: <5 mm in 5/22 (23%); 5–9 mm in 14/22 (64%); and ≥10 mm in 3/22 (14%) patients. There were four periprocedural complications (including one stroke and one intraprocedural rupture), none associated with neurological dysfunction. Angiographic follow-up was available in 18/22 (82%) and clinical follow-up in 19/22 (86%) patients, both at a median of 1 year (mean 1.2 years) after coiling. Aneurysm occlusion was complete in 12/18 (67%), a neck remnant was present in 3/18 (17%) and persistent aneurysmal filling was present in 3/18 (17%) patients, requiring retreatment in 1/18 (6%) patient. In-stent stenosis of 50%, which was asymptomatic, occurred in 1/18 (6%) patient. No subarachnoid hemorrhages and no ischemic events related to the procedure were observed during follow-up.
Conclusion In this small series, the technical success rate was 96%, there were no transient or permanent neurological complications and complete aneurysmal occlusion was achieved in two-thirds of treated aneurysms on follow-up angiography. These results suggest that in appropriately selected patients, stent assisted coil embolization of MCA aneurysms can be performed with a high degree of safety and acceptable durability.
Statistics from Altmetric.com
Introduction
Middle cerebral artery (MCA) aneurysms have traditionally been considered to be more amenable to surgical clipping than endovascular coiling. Surgically, exposure of MCA aneurysms is frequently straightforward and allows for accurate visualization of all major branches. Nonetheless, in addition to the ischemic and hemorrhagic complications common to both surgical and endovascular approaches, the surgical approach and associated brain retraction may lead to seizure,1 postoperative epidural hematoma and difficulties with wound healing.
Many of these issues may be avoided by endovascular treatment, but MCA aneurysms frequently pose challenges for coil embolization without assist devices due to wide necks, incorporation of significant branches and overlapping vasculature on angiography. With the advent of assist devices such as balloons and intracranial stents, aneurysms previously untreatable by endovascular approaches can increasingly be effectively occluded by coil embolization.2 Despite increased interest in coil embolization of MCA aneurysms, published experience with stent assisted coil embolization is limited to only a handful of small case series.3–6 We therefore retrospectively reviewed the experience with stent assisted coil embolization of unruptured MCA aneurysms at our institution.
Methods
Data collection
This was a retrospective, single center, consecutive case series. The study was approved by the institutional review board. All patients treated with Neuroform stents (Boston Scientific, Natick, Massachusetts, USA) between January 2004 and April 2009 were identified by billing records. Medical charts and imaging were then reviewed to identify patients undergoing coil embolization of MCA aneurysms. The following variables were collected: clinical features (age, history of subarachnoid hemorrhage), aneurysm features (aneurysm location (classified as proximal, bifurcation or distal), aneurysm size), procedural details (stent dimensions, degree of occlusion of aneurysm—classified according to the Raymond classification as complete, neck remnant or residual filling of the aneurysm7, procedural complications (thromboembolism, subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage, transient neurologic dysfunction, permanent neurologic dysfunction, major bleeding, pseudoaneurysm), clinical follow-up (delayed ischemic complications, SAH) and angiographic follow-up (degree of occlusion at last angiogram, retreatment).
Patient selection
Diagnostic angiography with three-dimensional reconstruction was performed prior to treatment in all patients. All patients with MCA aneurysms referred to the interventional neuroradiology service for diagnosis and treatment were considered for endovascular treatment. Aneurysms <5 mm were treated only if indicated by a family history of a first degree relative with SAH or if a ruptured aneurysm was present at another site. Informed consent was obtained from all patients, including discussion of both open surgical or endovascular approaches. If endovascular treatment was chosen, stent assisted coil embolization in particular was pursued if: (1) the neck to dome ratio or the branching pattern required an assist device; (2) on diagnostic angiography, an angiographic embolization view could be obtained such that the aneurysm neck and all the major branches could be visualized without being superimposed on each other; (3) only two branches arose from the aneurysm neck (trifurcation aneurysms excluded); and (4) all branches in which a stent was to be placed (generally the M1 and an M2 branch) were 1.5 mm in diameter or more.
Antiplatet and anticoagulation
All patients were loaded with aspirin and clopidogrel prior to intervention (aspirin 325 mg and clopidogrel 75 mg daily for 5 days). Prior to the interventional portion of the procedure, patients were given heparin 5000 U and additional heparin as necessary to maintain an activated clotting time above 250 s. Heparinization was not reversed at the conclusion of the procedure. After the procedure, aspirin 325 mg was continued for 6 months and clopidogrel 75 mg for 3 months. This regimen was adopted during our early experience with Neuroform stents in which shorter durations of antiplatelet therapy were associated with delayed cerebral ischemic events.
Procedure
All cases were performed under general anesthesia. In most cases, a 6 F Flexor shuttle sheath (Cook Medical, Bloomington, Indiana, USA) was placed within the cervical internal carotid artery. At our institution, the 6 F Flexor is the standard guide catheter chosen for intracranial interventions in the anterior circulation because it provides relatively robust support with a low incidence of dissection. An SL 10 (for small aneurysms) or BSCI 1018 (for larger aneurysms) microcatheter (Stryker, Kalamazoo, Michigan, USA) was then navigated over a Synchro II 0.14 microwire (Stryker) through the M1 MCA into the M2 branch most likely to result in protection of both branches and neck reconstruction after stent deployment. The microcatheter was then exchanged for a Neuroform stent (Stryker) over a 300 cm Transend Ex exchange wire (Stryker). Stents were sized either to be slightly or significantly larger than the parent artery, with diameter chosen to maximize protection of the MCA branches related to the aneurysm. The SL10 or BSCI 1018 microcatheter was subsequently navigated over the Synchro II microwire into the distal one-third of the middle of the dome of the aneurysm. The stent was then deployed across the neck of the aneurysm, jailing the microcatheter within the aneurysm. Coils were deployed until they either could not be placed without prolapse into the parent artery or without pushing the microcatheter out of the aneurysm.
Follow-up
Patients were discharged the morning following the procedure. Clinical follow-up was scheduled for 2 weeks after the procedure. Follow-up conventional angiography and clinical evaluation were scheduled for 6 and 12 months after the procedure. Afterward, if the aneurysm remained persistently occluded, patients were followed with MRI/MR angiography.
Results
Stent assisted coil embolization of the MCA was attempted in 23 patients and was successful in 22 (96%). In one case, the stent could not be delivered due to tortuosity and consequently the patient was subsequently referred for surgical clipping. The precise percentage of patients referred for coil embolization but deemed unsuitable due to anatomic factors cannot be determined. We estimate that 30% of MCA aneurysms referred for potential endovascular treatment were unsuitable based on the initial diagnostic angiogram, and an additional 10% were treated endovascularly without the use of a stent (either with balloon remodeling or without the use of an assist device). The most common reason for the decision not to pursue endovascular treatment was the inability to obtain an adequate embolization view based on the three-dimensional angiogram due to obscuration of the aneurysm neck by MCA branches or by more proximal vessels (M1 MCA, internal carotid artery or any other arteries overlapping the neck on the embolization view). As mentioned above, only MCA bifurcation aneurysms were treated and MCA trifurcation aneurysms were excluded.
Patient demographics, aneurysm features and procedural details are shown in table 1. All treated aneurysms were unruptured. Two patients had SAH due to ruptured aneurysms at a different location. The majority (20/22; 91%) of treated aneurysms occurred at the MCA bifurcation. At the conclusion of the procedure, complete occlusion was achieved in 41%, residual neck remained in 27% and persistent filling of the dome was observed in 32%.
Demographics and procedural details
A total of four procedural complications occurred, none of which resulted in either transient or permanent neurological deficits. Three periprocedural ischemic events occurred, all asymptomatic. In one patient, in whom stent placement was difficult (the stent could not be pushed out of the delivery catheter in the standard fashion and required deployment with a 0.025 glidewire (Terumo, Tokyo, Japan)), an M2 branch was occluded. Although an infarct was seen on CT, the patient did not exhibit clinical symptoms. In a second patient, on control angiography, a thrombus at the base of the coil mass was visualized; this was treated with abciximab 0.25 mg/kg intravenous bolus and intra-arterial tissue plaminogen activator 5 mg, with partial resolution but did not lead to a stroke either clinically or radiographically. A third patient had slow flow through an M2 MCA branch, presumably resulting from the stent; this was not treated and was seen to worsen on the first follow-up angiogram and then improve on the second. One intraprocedural hemorrhage occurred early in our experience due to guidewire manipulation just prior to placement of the first coil, resulting in mild contrast extravasation and SAH; this event was asymptomatic except for headache.
Angiographic and clinical follow-up were available in 18/22 (82%) and 19/22 (86%) patients, respectively (see table 2). Three patients were lost to follow-up and could not be contacted (14%); one patient was evaluated clinically by a stroke neurologist but never underwent follow-up angiography.
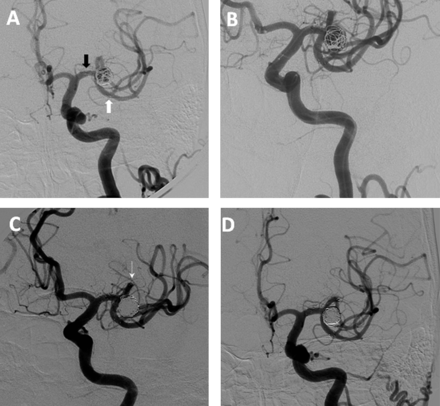
At a mean follow-up period of 12 months after the initial treatment, aneurysm occlusion was complete in 12/18 (67%) patients, a neck remnant remained in 3/18 (17%) and residual aneurysmal filling was present in three (17%) patients. From the initial post-procedure control angiogram to the last follow-up angiogram, 50% of aneurysms exhibited progressive thrombosis, 39% were stable and 11% developed recurrence with enlargement of the aneurysm at the base. Only one patient required retreatment, which was performed by repeated stent assisted coil embolization resulting in a Y stent configuration (see figure 1).
Clinical and angiographic follow-up
Illustrative case. (A) Control angiogram after initial stent assisted coil embolization of a 10 mm left middle cerebral artery (MCA) aneurysm, demonstrating persistent aneurysmal filling. The stent traverses the M1 into the inferior division of the MCA; the black arrow indicates the location of the proximal tines of the stent and the white arrow indicates the location of the distal tines. (B) Follow-up angiography at 6 months after initial treatment, demonstrating enlargement of the aneurysm with progressive recanalization. (C) Control angiogram following stent assisted embolization of the recurrent aneurysm with complete aneurysmal occlusion. The second stent traverses the M1 into the superior division of the MCA. The arrow indicates the location of the distal tines of the second stent. (D) Follow-up angiography at 1 year after retreatment showing persistent complete occlusion.
One patient (1/18; 6%) developed asymptomatic 50% stenosis of the stent on routine angiographic follow-up at 5 months after embolization which was stable on subsequent angiography at 13 months after the procedure.
Two patients (2/19; 11%) had possible cerebral ischemic events, both unlikely related to the aneurysm treatment. One patient developed transient dysarthria and dizziness 4 months after embolization. MRI showed no ischemia and the etiology was unclear but was not felt to be ischemic. The other patient had a right MCA bifurcation aneurysm which was treated with a stent from the distal M1 to M2; 2 months after treatment, he developed a right basal ganglia lacunar stroke visualized by diffusion weighted MRI. Because the proximal aspect of the stent was placed distal to the origin of the lateral lenticulostriates from the M1 MCA, the stroke neurology team determined that this event mostly likely was the result of small vessel disease and was unrelated to the stent.
Discussion
In this small, single center, retrospective series, stent assisted coil embolization of MCA aneurysms appeared to be a promising alternative to open neurosurgical clipping in properly selected patients. The technical success rate was 96%. There were no transient or permanent neurological complications although a clinically silent stroke due to a branch occlusion was visualized on a post-procedure CT and an intraprocedural rupture without clinical consequences occurred. Complete aneurysmal occlusion was achieved in two-thirds of treated aneurysms on follow-up angiography. No delayed thromboembolic events attributable to stent placement were detected. Asymptomatic in-stent stenosis of 50% occurred in one patient. Complications which may result from open surgery—including epidural hematoma, seizure and wound infection—were not observed in this patient population.
The technique of stent assisted coil embolization offers a number of potential advantages. Stenting facilitates the treatment of wide necked and small aneurysms by creating a barrier to prevent coils from herniating into the parent artery, decreasing the risk of parent artery occlusion and possibly increasing coil packing density. Other potential advantages include altered flow dynamics and promotion of thrombosis. Finally, stents may serve as a framework for endothelialization.3 ,8–10
Several centers have reported case series of patients with MCA aneurysms treated with endovascular coil embolization, although stent assisted embolization was not employed in the vast majority of these interventions. In the largest published series of endovascular treatment of MCA aneurysms, 174 patients (36% unruptured; 64% ruptured) underwent attempted coil embolization, and successful embolization was accomplished in 160 patients (92%). The risk of procedure related disability or death was 5.7% (2.1% severe infarction; 1.4% death).11 A second group recently reported the results of MCA coil embolization in 115 patients (42% ruptured; 58% unruptured). Complications were seen in 9%, including thromboembolic complications in 7%.12 In these two large series, none of the patients underwent stent assisted coil embolization.
Thus far, reports of stent assisted coil embolization of MCA aneurysms have been limited to a few small series3–6 and a handful of additional cases.9 ,13 The largest case series describes the results of stent assisted coil embolization of 50 aneurysms (47 patients). The technical success rate was 96.2%. In this case series, in-stent thrombosis was common, occurring in 20% of patients, perhaps because platelet inhibition assays were not used and patients were loaded with clopidogrel on the day prior to the procedure but aspirin was not administered until the conclusion of the procedure. Mortality and permanent neurologic morbidity were 0% and 4.3%, respectively.6 In another single center case series, 13 patients were treated with stent assisted coil embolization and three with primary stenting without coiling (5/16 unruptured; 10/16 ruptured; 1/16 recurrent). The only complication—a branch occlusion—did not result in clinically significant cerebral ischemia. No in-stent stenosis or aneurysm recanalization occurred at follow-up.5 In a case series of endovascular treatment of MCA aneurysms, 13/76 (17%) were treated with stent assisted coil embolization; one patient developed a thromboembolic complication with complete recovery. Although stent assisted coil embolization was used in a minority of cases in this series, assist techniques (including the dual catheter technique, balloon remodeling or stents) were required in 45/76 (59%).4
Our experience, in combination with that of other investigators, suggests that in properly selected patients, stent assisted coil embolization of MCA aneurysms can achieve periprocedural complication rates equivalent to open surgery.3–5 Surgical morbidity and mortality for MCA aneurysms has been reported to be approximately 3–4% for unruptured aneurysms although these series lack independent neurological assessment.14 ,15 In our series and in two others detailing the results of stent assisted embolization at the MCA, there were no deaths and no permanent neurologic deficits due to procedural complications4 ,5; as described above, in the largest case series, a 4.3% risk of permanent neurological morbidity was observed.6
It is important to note that the promising results seen with endovascular treatment of MCA aneurysms in our series as well as in other published series likely reflect careful patient selection, increasing experience and improving technology. As described, safe treatment requires an embolization view without superimposed vessels overlying the aneurysm neck and failure to achieve such a view is the most common reason for the decision not to pursue endovascular treatment. In addition, we generally exclude patients for treatment in whom the aneurysm involves an MCA trifurcation and avoid stents when the distal aspect of the stent will be placed in a vessel <1.5 mm in diameter. Additional factors contributing to decreased complication rates include increased experience and improvements in the trackability of successive generations of the Neuroform stent. We recently reported the results of stent assisted coil embolization of anterior communicating artery aneurysms treated at our institution; in our early experience (which occurred prior to the treatment of the MCA aneurysms described in the present investigation), we found substantially higher rates of morbidity and mortality.16 The Neuroform EZ stent (Stryker) became available after the present investigation was completed. We have found this stent to be easier to deploy accurately than the Neuroform 3 (Stryker) for standard anatomy; because of the increased stiffness of the 027 inch delivery catheters and decreased trackability of the stent itself relative to the Neuroform 3, however, we continue to employ the Neuroform 3 for extremely tortuous anatomy.
Although the periprocedural outcomes and technical success rates with stent assisted coil embolization of the MCA appear promising, the long term durability of coil embolization in relation to surgical clipping remains relatively unknown. Among the nearly 400 patients described in the two reports of embolization of MCA aneurysms without stent assist, with 50 months follow-up in the series of 174 patients and 16 months in the series of 115 patients, there were only two instances of rebleeding of a treated aneurysm (both previously ruptured).11 ,12 Among the smaller group of MCA aneurysms treated with stent assist, none ruptured during follow-up. Nonetheless, aneurysm recurrence remains a significant concern. With coil embolization of MCA aneurysms without stenting, aneurysm recurrence rates of up to 27% at late follow-up have been reported.11 ,12 Some evidence suggests that stent assisted coil embolization may lead to more durable results due to progressive thrombosis.3 ,5 ,9 ,10 In our cohort, 50% of aneurysms exhibited progressive thrombosis, and progressive recanalization occurred in only 11%, consistent with the relatively low rates of aneurysmal recanalization seen in other series of stent assisted coil embolization of MCA aneurysms.3–5
Our investigation has a number of limitations. This was a single center retrospective series and is therefore subject to the limitations of this research design, including selection bias, lack of independent verification of outcomes and ascertainment bias arising from the lack of prospectively collected data on procedural and clinical outcomes.
Conclusions
Stent assisted coil embolization appears to be a promising technique for the treatment of selected unruptured MCA aneurysms. We achieved a technical success rate of 96% without any transient or permanent neurological complications. Complete aneurysmal occlusion was present in two-thirds of treated aneurysms on follow-up angiography. There were no delayed thromboembolic complications attributable to stenting, and asymptomatic in-stent stenosis of 50% occurred in only one patient. These results suggest that in selected patients, stent assisted coil embolization of MCA aneurysms can be performed with a high degree of technical success, a low procedural risk and acceptable durability. Furthermore, our results in combination with other recent reports3–6 suggest that direct comparison of endovascular and open surgical approaches to the treatment of unruptured MCA aneurysms may be warranted.
Key points
-
Middle cerebral artery (MCA) aneurysms have traditionally been considered to be more amenable to surgical clipping than endovascular coiling but the availability of stents designed for intracranial stent assisted coil embolization has increased the feasibility of the treatment of MCA aneurysms with endovascular techniques.
-
We retrospectively reviewed the procedural details and outcomes of stent assisted coil embolization in 23 consecutive patients treated at our institution.
-
Technical success was achieved in 22/23 with periprocedural complications in four patients (none associated with neurological disability); on follow-up angiography, 67% of patients had complete aneurysmal occlusion and only one patient required retreatment.
-
These results suggest that in appropriately selected patients, stent assisted coil embolization of MCA aneurysms can be performed with a high degree of safety and acceptable durability.
References
Footnotes
-
Competing interests None.
-
Ethics approval The study was approved by the Institutional Review Board, Oregon Health and Science University.
-
Provenance and peer review Not commissioned; externally peer reviewed.