Article Text
Abstract
Background Arteriovenous malformations (AVMs) of the brain are commonly treated in multimodality fashion, with endovascular embolization followed by surgical extirpation being one of the most effective strategies. Modern endovascular suites enable rotational angiography, also known as cone-beam CT angiography (CBCT-A), using the full capability of modern C-arm digital angiography systems. This imaging modality offers a superior image quality to current options such as digital subtraction angiography, MRI, or CT angiography. Preoperative planning can be greatly aided by the resolution of angioarchitecture seen in CBCT-A images. Furthermore, these images can be used for intraoperative neuronavigation when integrated with widely used frameless stereotactic systems. The utility and outcome of the use of CBCT-A for preoperative planning and intraoperative localization of AVMs was evaluated.
Methods A retrospective review was performed of 16 patients in which CBCT-A was performed, including radiological review and all clinical data.
Results CBCT-A was successfully employed in all cases including those with (n=9) and without (n=7) rupture. Complete resection confirmed by postoperative angiography was achieved in all cases.
Conclusions We present a novel application of CBCT-A in the treatment of AVMs, both for preoperative surgical planning and an intraoperative reference during neuronavigation.
- Arteriovenous Malformation
- Brain
- CT Angiography
- Vascular Malformation
- Angiography
Statistics from Altmetric.com
Introduction
Arteriovenous malformations (AVMs) of the brain are commonly treated in multimodality fashion, with endovascular embolization followed by surgical extirpation being one of the most effective strategies. Embolization can reduce blood loss and minimize complications associated with microsurgery for AVMs. Frameless stereotactic image guidance can also be used to make AVM resection safer. Image guidance has become standard in cranial neurosurgery, increasing the accuracy of craniotomy and allowing for smaller scalp incisions and bone flaps. Although image guidance for AVM surgery has been previously described in the literature, the optimal timing and imaging modality have not been established.
The capabilities of modern angiographic platforms have recently improved substantially. Two-dimensional (2D) digital subtraction angiography (DSA) can now be enhanced by 3D, functional, and axial-anatomic adjunctive technologies. One such technology, rotational angiography, also known as cone-beam CT angiography (CBCT-A), uses the full capability of modern C-arm digital angiography systems available in neuroendovascular suites. An angiographic imaging study immediately prior to surgical resection can provide important information to a cerebrovascular surgeon. In this study we review a series of cases in which CBCT-A was performed preoperatively, either with or without an endovascular intervention, to assist in the image-guided surgical resection of cerebral AVMs. The use of CBCT-A has been tested in laboratory/cadaveric studies by other groups;1 we report its use in a series of patients at two different institutions.
Methods
Patient data
The objective of the study was to retrospectively review demographic, clinical, and imaging data for all patients who underwent surgical treatment of their AVM with the aid of intraoperative image guidance using preoperatively obtained CBCT-A. All such AVM resections done by the senior authors (EAMD, AA) from 2011 to 2014 were retrospectively reviewed. Electronic medical records, including clinic and hospital records, as well as relevant imaging were analyzed.
Cone-beam CT in the neurointerventional suite
Most neuroendovascular interventions are facilitated by single-plane or biplane C-arm systems. Classically, diagnosis and treatment of vascular anomalies has relied on 2D vascular imaging for visualization. Yet recent advancements have seen a transition from systems employing image-intensifier detectors to flat-panel detectors (FPD).2–6 This transition has enabled rapid acquisition of 3D vascular images (3D DSA) as well as CBCT soft tissue imaging, with and without contrast agent present.2
The acquisition procedure for a C-arm based volumetric scan is as follows: first, images are acquired during a semicircular rotation around the patient, with image acquisition performed at discrete intervals (150–600 images depending on the target). Next, contrast agent is injected and the same acquisition procedure is repeated. The images are processed for detector effects (offset and gain correction) and physical effects (scattered radiation and X-ray beam hardening), and reconstructed using a modification of the filtered back projection (FBP) algorithm.5 Non-subtracted images yield CT-like images, while subtraction of the two runs with subsequent reconstruction yields a high-quality representation of the vascular anatomy.
Navigation using volumetric data acquired in the interventional suite
The volumetric data acquired in the interventional suite were exported to the navigation system in the DICOM format using a 512×512×396 matrix size and a homogeneous voxel resolution of 0.47 mm. The data were loaded onto the navigation system (StealthStation S7, Medtronic, Minneapolis, Minnesota, USA) using either PACS transfer or manual upload. Additional patient data from preoperatively obtained MRI or CT scanning were imported as well in some cases. The different datasets were registered using rigid registration. Following patient positioning and immobilization using a Mayfield clamp, skin line tracing registration was performed using either DynaCT or Stealth-protocol CT registration models. Approach planning as well as navigation was performed using the datasets from all modalities and the surgeon preference for each modality was noted after the case (figure 1). The minimal voxel resolution yielded by the systems employed (Artis Zee Biplane, Siemens Healthcare, Forchheim, Germany) was 0.46 mm (homogenous voxel side length). Neither navigation nor initial data fusion in the navigation system was impeded.
(A) StealthStation navigation pane as used in preoperative planning. The views are dynamically customizable and allow for different views to be viewed in different phases of the surgical process. The use of the three-dimensional scalp view allows for incision planning, while the views in (B) are best for craniotomy planning. (B) Intraoperative navigation pane presented to the surgeon. The cross-sectional slice views (left top/bottom) allow navigation through the complex vasculature with respect to the patient's anatomy and the trajectory views (right top/bottom) allow visualization of the anatomy traversed during dissection.
Results
Patient clinical demographics
Our series included 16 patients with AVMs treated at Baylor College of Medicine and Semmes-Murphey Neurologic and Spine Institute (table 1). Demographics, lesion characteristics, and treatment modalities are similar to modern published series of AVM management.7 Regardless of whether or not the patients received preoperative endovascular treatment for their AVM, all patients underwent a diagnostic angiogram and DynaCT prior to neurosurgical intervention. This included the nine patients in our cohort who presented with rupture, who also required urgent neurocritical care and intracranial pressure management such as ventriculostomy placement. The embolic agent of choice in most cases was Onyx 18; n-butyl cyanoacrylate (n-BCA) was also used primarily or as an adjunct in some cases (table 1).
Summary of patient, lesion, and treatment data for 16 patients with 16 treated AVMs at Baylor College of Medicine and Semmes-Murphey Neurologic and Spine Institute
Devised workflow
Patients underwent either staged or immediate preoperative embolization of their lesions. At the end of the final endovascular procedure a CBCT-A was obtained. In non-emergency cases the cerebrovascular surgeon had the opportunity to evaluate and analyze these images in 3D, with custom reconstructions showing lesional angioarchitecture as well as areas of embolization. This is shown in a flowchart (figure 2).
Workflow to incorporate three-dimensional (3D) angiographic imaging into a navigation system. Following catheter placement, high-quality 3D digital subtraction angiography (DSA) acquisition is performed capturing the head and the nose of the patient. The data acquired yield a soft cone-beam CT (CBCT) with opacified vasculature from fill acquisition, as well as a 3D DSA depicting the angioarchitecture. The data are transferred to the navigation system and vendor-specific image fusion to preoperative data is performed. The CBCT image is selected for skin line registration and navigation is commenced.
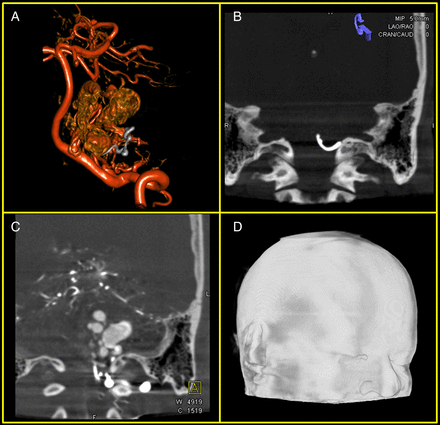
CBCT images were noted to have high spatial resolution and homogeneous sub-mm voxel size provided by the FPD-based C-arm system. With the systems used by the authors (Artis Zee Biplane and Artis Zeego, Siemens Medical Solutions), a dedicated scan protocol for neurosurgery yielded three volumes from one dual-spin CBCT acquisition with concurrent contrast agent injection: 3D-DSA, providing a high resolution image of the patient’s vasculature, allowing delineation of the nidus and other critical structures for AVM treatment; a CT-like image (DynaCT) displaying the position of injected embolic agent with respect to the patient’s anatomy; and a CT angiography (CTA)-like image giving information on the patient's vasculature and the injected embolic agent with respect to the patient's anatomy (DynaCTA) (figure 3A–C). In addition, this scan protocol allowed for an accurate representation of the patient's skin line for tracer-based registration on a stereotactic navigation system and focused on including the top of the head and tip of the nose to ease registration (figure 3D).
Datasets reconstructed from a single cone-beam CT acquisition procedure. (A) Three-dimensional digital subtraction angiography (DSA) of patient's vascular anatomy. The data have been acquired post-embolization of the arteriovenous malformation feeding vessels (the vasculature is displayed in red while the injected agent is displayed in gray). (B) DynaCT after injection of embolic agent. The maximum intensity projection (MIP) display (5.0 mm thickness) allows for delineation of the shape of the injected agent. (C) DynaCTA (CT angiography) after injection of embolic agent. The patient's vasculature with respect to the anatomy is well visualized. (D) Skin line representation of acquired DynaCT required for tracer-based patient registration in navigation system.
Patients were transferred from the endovascular suite to the operating room and their heads were fixed in a radiolucent Mayfield head-holder. CBCT images were then loaded into the frameless neuronavigation system (Medtronic StealthStation S7). Registration was performed using the ‘tracer’ method and confirmed with anatomical landmarks. For planning of scalp incision and craniotomy flap, the Medtronic passive planar blunt probe was used. Thereafter, the operating microscope was integrated and used as the navigated instrument. In our series there were two strategies for using the images obtained in the angiography suite; one author used a standard Stealth protocol CT merged with the DynaCTA images (figure 4) while the other author used the DynaCTA images alone. There did not appear to be a difference between these strategies with regard to the quality of images or accuracy of navigation. Most frequently, the CTA-like images in three planes were used for surgical navigation, with the 3D view as an adjunct (figure 4). Indocyanine Green (ICG) video angiograms were used to confirm resection of the AVM prior to closure in most cases, and all cases had postoperative completion angiography to assess for completeness of resection (table 1). Complete resection was confirmed by postoperative angiography. In two cases abnormal vasculature was identified on postoperative angiography. Perinidal hypervascularity is a common finding and must be distinguished from true AVM nidus both preoperatively and on postoperative angiography. We preoperatively did not intend to resect the areas identified on the postoperative angiograms, and our presumption is that these areas will normalize radiographically over time. No shunting was exhibited on any postoperative angiogram.
Examples of (A) MRI and (B) DynaCT with three-dimensional reconstruction for intraoperative navigation. Here, orthogonal views are presented (axial, coronal, sagittal), but trajectory views are most useful. Due to various artifacts, MRI has poor resolution of the angioanatomy. Compared with the traditional angiogram, DynaCT offers additional resolution of soft tissue and bony anatomy.
Discussion
The modern treatment paradigm for many AVMs now entails a combination of preoperative embolization followed by neurosurgical excision, especially those with Spetzler–Martin grades 3 or B.8 In our series of 16 patients with AVMs treated at two institutions, CBCT-A allowed for safe and accurate surgical extirpation following evaluation and treatment in the angiography suite.
AVMs can be assessed preoperatively and intraoperatively with a wide variety of imaging modalities including CT, CTA, MRI, and DSA. Each of these modalities has drawbacks, including the time necessary to obtain them. MRI visualization of AVMs often shows a pattern of flow voids9 and allows assessment of brain parenchyma and proximity to eloquent cortex. MRI yields comparatively little information about angioarchitecture and blood flow, but excels in gyral and sulcal definition and is the best modality for identification of potentially eloquent parenchymal structures. Furthermore, MRI can be time-consuming and may misrepresent qualities of the nidus,10 as artifact from dense embolisate such as Onyx or n-BCA can cause significant image degradation. CTA can provide high quality imaging of large vessels, but visualization of small intracranial vasculature and complex pathological anatomy (such as AVMs) remains challenging. This is due to comparably low spatial resolution (∼1 mm maximally), differentiation challenges near bony anatomy due to non-subtracted imaging, and the lack of vessel-selective imaging.11 CTA also suffers from a lack of time resolution, making angiographic evaluation invaluable for a comprehensive understanding of the flow patterns that characterize many of the potential symptoms and complications associated with the natural history and treatment of AVMs.
The combination of DSA and CBCT-A, on the other hand, uses the full capability of modern C-arm digital angiography systems. Such imaging provides crucial information on an AVM, its angioarchitecture and relationships to bone and surrounding brain structures, and has distinct advantages over the other imaging modalities.12 ,13 Previous authors have described and compared the effectiveness of CBCT-A against DSA in assessing AVM dimension, arterial feeders, venous drainage, nidal density, and shunting;14 all of this information is further provided in three dimensions instead of two.15 Furthermore, CBCT-A overcomes the limitations of DSA with use in neuronavigation systems by acquisition of skin line tracings, allowing it to be used as a stand-alone reference image.1 CBCT-A has also been shown to be useful in the assessment of AVMs following hemorrhage, especially when DSA does not sufficiently show the lesion.12 Specifically, CBCT-A allows for accurate assessment of the AVM nidus for optimal treatment planning, whether during the preoperative neuroendovascular phase of treatment or during microsurgical intervention.16–18 Our use of a low-dose contrast injection while simultaneously repeating the rotational scan allowed subtraction of the radially distributed projection images and subsequent reconstruction of a high-detail volumetric image of the patient's vasculature. This was useful during preoperative planning and intraoperatively. The CTA-like (CBCT-A) images which were reconstructed were loaded into the image guidance system allowed for real-time accounting of the progress of dissection.
It should be noted that the benefits of CBCT-A do not render traditional DSA obsolete nor supplant it as the gold standard. Instead, CBCT-A functions as an extremely useful adjunct that is most commonly acquired following DSA, offering its own unique strengths. MRI can be (and was) fused with CBCT-A images in our series and provided important contextual brain anatomy, especially for AVMs close to eloquent areas.
Hybrid operating rooms have become popular in recent years, allowing both endovascular interventions and traditional neurosurgical procedures to occur without patient transfer,19 often by the same comprehensive cerebrovascular surgeon. These rooms have the capability of rapid imaging and assessment of the lesion, as well as providing a sterile field for open surgical intervention. In the presented series, CBCT-A and interventions were performed in an endovascular suite separate from the operating room, but our experience with integration of DynaCTA images in neuronavigation suggests that this technology and strategy may be even more seamless in hybrid neurovascular suites. With the benefit of avoiding patient transfer, total operative times can be shortened. Furthermore, intraoperative DynaCTA images could be obtained to allow for updated navigation, to confirm excision, and upon closing to assess for a hematoma. In a hybrid suite, with maintained microsurgical exposure, post-resection confirmation of complete exclusion of the lesion could occur at a far higher resolution than traditional fluoroscopy-based methods.15 ,20
The use of this technology and resulting workflow created several evident advantages. Intraoperative differentiation between embolized areas of the AVM and active areas seen via the microscope were confirmed on the DynaCTA navigation. Furthermore, in those cases treated soon after rupture, it enhanced identification of the lesion within the hematoma. Approach to the deep borders of the malformations was greatly aided, minimizing the need for excessive corticectomy. The extent of resection was probably improved with the aid of DynaCTA and could perhaps be further improved with its use in a hybrid neurovascular suite.
The care of cerebrovascular patients is becoming more integrated in terms of treatment strategies, location of care, and even providers. Potential areas for further development include better integration of the radiographic images and reconstructions with the microscope via more advanced heads-up displays. The enhancement of image quality by a variety of post-processing techniques has progressed significantly in recent years and should continue. Examples include metallic artifact reduction and region of interest analysis, which can be combined to produce remarkably vivid images despite the presence of dense metal objects.21–23 These emerging technologies available with Siemens and other angiography equipment can help visualize AVMs and differentiate residual lesion and embolisate.
Limitations
There are notable limitations to CBCT-A, but it is not intended to supersede the gold standard, conventional angiography. Other imaging modalities such as MRI provide anatomical context, and are synergistic with CBCT-A through fusion of DICOM images in neuronavigation systems. Most of the AVMs in our series were fed from a single circulation, especially after embolization, and the CBCT-A images were created by injection within a single vessel. Vascular malformations fed by both anterior and posterior circulations, internal and external branches, or bilateral feeding require special consideration. We are currently working with an angiography platform manufacturer on rotational angiography fusion techniques to allow for improved visualization of multiple circulations and radiodense embolic agents using color differentiation. Fusion and post-processing may thus obviate the need for dual catheter injections which increase the risk, complexity, and time of an angiographic procedure.
Nidus that is distal to embolized arteries may be incompletely visualized by CBCT-A following embolization because contrast will not flow into it. MRI is a useful modality for identifying residual nidus in these cases, and was used as an adjunct in most of our cases. Many cases were also imaged intraoperatively with indocyanine videoangiography, which can identify areas of the surgical bed that may deserve closer inspection to rule out the presence of residual nidus. ICG was used in most cases, based on the intraoperative specifics of each surgery. There are well-described limitations of ICG videoangiography in AVM surgery,24–26 but it can still serve as a useful adjunct—for example, to ensure complete obliteration of feeding to a nidus prior to dividing the main draining vein. One of the most important pitfalls that should be noted, however, is the difficulty in seeing beyond dissected parenchyma to identify residual nidus. Large residuals will illuminate through thin layers of parenchyma but small residuals may very well not be identified. Intraoperative angiography in a proper suite is valuable, a point that should be acknowledged, but is not routinely necessary and carries some risk. Intraoperative DSA, possibly with CBCT-A, in a hybrid operating room may represent the ideal solution in the future.
Conclusion
We present a novel application of CBCT-A in the treatment of AVMs, both for preoperative surgical planning and an intraoperative reference during neuronavigation. The additional resolution of AVM angioarchitecture provided by this imaging as well as the efficient workflow inherent in the technology hold promise for further and better integration of the traditional dichotomy (‘open’ and ‘endovascular’) in cerebrovascular treatments.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online tables
Footnotes
Contributors Study design: AA, EAMD. Data collection, composition of manuscript: VMS, SS. Data review, statistical analysis: VMS. Editing: all authors. Approval of manuscript: all authors.
Ethics approval Ethics approval was obtained from the hospital IRB.
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests SS is a paid employee of Siemens Healthcare USA, the manufacturer and developer of the C-arm systems used in this study.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Technical summary and further information regarding our research are available from the corresponding author at the Baylor College of Medicine. Any transmission of this data must be approved by the Baylor College of Medicine and Semmes-Murphey Neurologic and Spine Institute.