Article Text
Abstract
Background Aneurysmal subarachnoid hemorrhage (aSAH) secondary to blister-type aneurysms (BAs) is associated with high morbidity and mortality. Microsurgical clipping or wrapping and/or use of traditional endovascular techniques to repair the lesion result in frequent regrowth and rebleeds and ultimately high fatality rates. Because of the purely endoluminal nature of arterial reconstruction, flow diversion may represent an ideal option to repair ruptured BAs.
Methods We performed a retrospective analysis of our database including all consecutive patients with aSAH secondary to BAs treated with the Pipeline Embolic Device (PED) between November 2013 and November 2015 in two institutions. We collected basic patient demographics, aneurysm size, location, number and sizes of PEDs used, use of coiling, 30-day modified Rankin Scale (mRS) score, and follow-up imaging data.
Results Ten cases of aSAH were found as a result of a ruptured BA. Patients had a mean age of 47.2 years (range 27–68). Mean Hunt and Hess score was 1.6 (range 1–4). Lesions were predominantly left-sided, mostly along the dorsal aspect of the internal carotid artery, either paraclinoid or paraophthalmic (8/10). In two patients the BA was located in the left middle cerebral artery. All lesions were very small (mean 1.4×1.5 mm; range 0.75–2.1 mm). Placement of a single PED resulted in immediate occlusion or near-occlusion of the BA in 9 out of 10 patients. Nine patients did very well; eight had a 90-day mRS score of 0 and one had a 90-day mRS score of 1. Follow-up digital subtraction angiography was performed in all patients (mean 15 months; range 7–24). In the surviving nine patients there was complete occlusion of the BA on long-term follow-up angiography.
Conclusions Repair of ruptured BA with PED may be a safe and durable option.
- Aneurysm
- Flow Diverter
- Subarachnoid
- Hemorrhage
Statistics from Altmetric.com
Blister-type aneurysms (BAs) were initially described in 1986 as aneurysms arising from the dorsal aspect of the internal carotid artery (ICA).1 Since the initial description as ICA aneurysms, they have been reported in the middle cerebral artery (MCA), anterior cerebral artery, anterior communicating artery, basilar artery, and posterior inferior cerebellar artery.2 ,3 Presentation is typically with acute subarachnoid hemorrhage (aSAH) related to aneurysmal rupture.
On digital subtraction angiography (DSA) they appear as very small (1–2 mm) irregularly-shaped aneurysms with a wide or non-discernable neck. Pathologically, BAs are very different from saccular aneurysms.4 Histological examination shows abrupt loss of both the intima and media.4 ,5 As a result, the outer wall of BAs is comprised of only adventitia and/or thrombus, often adherent to the overlying pia, essentially with the body of the aneurysm behaving like a pseudoaneurysm with no true wall structure. Because of such extreme fragility of the entire BA arterial histoarchitecture, we do not currently have safe, effective, and durable options to repair these aneurysms. Manipulation of the lesion either by microsurgical clipping or wrapping and/or traditional endovascular techniques using coils with adjunctive balloons or stents results in rupture of the artery, regrowth, and rebleeding of the aneurysm and ultimately high fatality rates.6 Additional endovascular approaches have included endovascular deconstruction and sacrifice of the affected vessel; however, with frequent high-grade SAH, younger age of patients and concerns for vasospasm and secondary inadequate collateral circulation, deconstructive strategies are at best poor alternatives. A conservative approach by non-treatment of these lesions results in growth and high rates of rebleeds, typically within the first week after the initial SAH.7
In contrast to microsurgical clipping or endovascular coil embolization, the implant of a flow diverter (FD) allows progressive aneurysmal occlusion with remodeling of the arterial lumen with minimal manipulation of the aneurysmal sac.8 Flow diversion results in reduced hemodynamic stress/velocity within the body of the aneurysm, which should result in a reduced risk of re-rupture and better long-term occlusion. The concern remains the need for dual antiplatelet therapy and for additional invasive procedures. The Pipeline Embolic Device (PED; Medtronic, Irvine, California, USA) is a self-expanding FD made of a braided multi-alloy cylindrical mesh. With regard to safety and effectiveness, there are published series of over 1000 cases worldwide on the treatment of large and giant ICA aneurysms with PED (PUFF, INTREPeD). Because of the purely endoluminal nature of aneurysmal occlusion, PED may represent an ideal option to repair ruptured BAs. Previous multicenter small case series seem to indicate that PED may be an option in these cases.9 ,10
We report a two-center experience on the endovascular treatment by PED of patients with aSAH secondary to BA.
Methods
Data collection
Patient data collection was approved by the Institutional Review Boards of both institutions.
Patient population
We performed a retrospective analysis of our database including all consecutive cases of patients with aSAH secondary to BA between November 2013 and November 2015 at Baptist Hospital in Miami, Florida and Gates Vascular Institute and SUNY University at Buffalo in Buffalo, New York.
All patients with a diagnosis of aSAH had conventional DSA including three-dimensional (3D) reconstructed images at the time of admission. Treatment of the aneurysm was based on each operator preference, including timing of endovascular device (EVD) placement, number of PEDs used, administration and dose of antiplatelet agents such as aspirin, clopidogrel, and glycoprotein IIb/IIIa inhibitors, and perioperative management of the patients. The timing and modality of follow-up postoperative imaging were also dependent on the operator's preference and the clinical status of the patient. We collected basic patient demographics, aneurysm size, location, number and size of PEDs used, use of coiling, 30-day modified Rankin Scale (mRS) score, and follow-up imaging data. Position and immediate angiographic result was recorded with conventional DSA images with or without XPER CT (Phillips Healthcare, Best, The Netherlands) and Low Contrast Imaging (Toshiba Medical Systems). Patients were admitted to the intensive care unit following treatment for further observation and management. Clinical outcomes included mRS. Imaging follow-up was performed with conventional DSA in all cases.
Results
In the study period in both institutions we treated a total of 255 aneurysms (73 ruptured). Ten cases of aSAH were found as a result of a ruptured BA in the two institutions (1.9% of all aneurysms and 6% of ruptured aneurysms; table 1). Patients tended to be young with a mean age of 47.2 years. The mean Hunt and Hess (H&H) score was 1.6 (range 1–4) and the mean Fisher grade was 2.3 (range 1–4). Lesions were predominantly left-sided (8/10), mostly located along the dorsal aspect of the ICA within either the paraclinoid or paraophthalmic segments. In two patients the BA was located in the left MCA. All lesions were positively identified on the initial DSA investigation when 3D reconstructions were employed (figures 1⇓–3). The lesions were all very small and wide-necked, protruding an average of 1.4×1.5 mm (range 0.75–2.1 mm) from the parent vessel (table 1).
Summary of patients' clinical features, outcomes, size and number of PED used
Case 1: (A) Lateral digital subtraction angiogram (DSA) on post-admission day (PAD) 0 showing a shallow 1.8 mm blister-type aneurysm along the dorsum of the right internal carotid artery (ICA). (B) Three-dimensional DSA on PAD 0 confirming right ICA blister aneurysm. (C) VCT on PAD 2 showing persistent filling of blister-type aneurysm following placement of 4.5×14 mm Pipeline flow diverter. (D) Lateral DSA on PAD 7 showing persistent filling of blister-type aneurysm and multifocal segmental vasospasm within the left anterior circulation following placement of two additional Pipeline flow diverting devices. The patient died on PAD 10.
Case 2: (A) Digital subtraction angiogram (DSA) showing shallow blister-type aneurysm along the lateral aspect of the LMCA. (B) DSA slowing reduced filling of LMCA aneurysm after placement of a 3×14mm pipeline flow diverter. (C) Expert CT prior to discharge showing no filling of blister-type aneurysm. (D) DSA on 6-month follow-up showing occlusion of aneurysm.
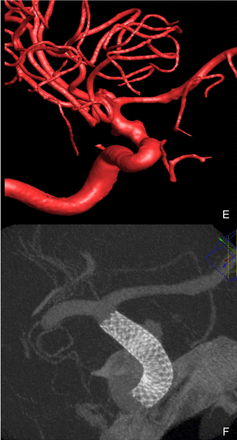
Case 4: (A) Lateral digital subtraction angiogram (DSA) on post-admission day (PAD) 0 showing shallow 1×3 mm blister-type aneurysm along the dorsal aspect of the left internal carotid artery (ICA). (B) Three-dimensional (3D) DSA on PAD 0 showing shallow blister-type aneurysm of ICA. (C) Lateral DSA after placement of Pipeline flow diverter showing residual filling of blister-type aneurysm. (D) Lateral DSA 1 year and 5 months following Pipeline placement showing no residual filling. (E) 3D reconstruction of the aneurysm. (F) Cone-beam CT after pipeline placement.
All patients were started on dual antiplatelet therapy prior to placement of the PED. The protocol and doses used were slightly different in Baptist Hospital and Buffalo University. Technical success with PED placement was achieved in all cases and there were no intraprocedural complications including vessel wall injury or thrombus formation.
Placement of a single PED resulted in immediate occlusion or near-occlusion of the BA in nine of the 10 patients. In one patient the lesion remained patent and tended to grow despite placement of three PEDs in two procedures. There was, however, no re-rupture in this case despite a dual antiplatelet regimen and growth of the lesion. The patient was admitted with H&H grade 4 and a large SAH (Fisher grade 4). He also developed severe vasospasm, requiring multiple sessions of intra-arterial interventions. The patient never regained consciousness. He was made comfort-care by the family and eventually died 10 days after the initial hemorrhage. The remaining nine patients did very well. Eight patients had a mRS score of 0 at 90-day follow-up and one had a 90-day mRS score of 1. Long-term follow-up imaging was performed by conventional DSA in eight of the nine patients. The average time of the follow-up DSA after PED placement was 15 months (range 7–24). In one patient the 6-month follow-up DSA was not performed. In the surviving nine patients there was complete occlusion of the aneurysm on long-term follow-up angiography.
Discussion
In the present series of 10 patients with aSAH secondary to ruptured BA treated with PED implant, nine had excellent outcomes (90-day mRS 0 or 1). One patient died as a consequence of the original massive SAH and subsequent severe vasospasm. Long-term follow-up by DSA demonstrated complete occlusion of the BA and remodeling of the artery without recurrence or recanalization of the aneurysm.
Ruptured BAs are relatively rare, comprising 0.9–6.5% of ICA aneurysms and <1% of all intracranial aneurysms.11 It is estimated that 2.2% of cases of SAH from ICA aneurysms are the result of ruptured BAs.7 These lesions tend to occur more frequently in women and are associated with younger age, hypertension, and ICA atherosclerosis or dissection.7 Because of their very small size, they are difficult to detect on initial CT angiograms or even on DSA.12 These lesions may increase in size in the few days or weeks following rupture. Therefore, if not detected on the first DSA, they are often detected on follow-up angiograms.
Because of the abrupt disruption of the architecture of the arterial layers, BAs are fragile and prone to spontaneous rebleeds. In addition, the histological features of the BA and often of a large-cross section of the artery harboring the lesion are at high risk of rupture during surgical and endovascular manipulation. Surgical management in the past included microsurgical clipping or deconstructive approaches to the ICA or vessel trapping with or without high-flow bypass with poor outcomes.6 Ogawa et al,7 in a series of patients treated with a clip-wrapping technique, reported slightly better outcomes compared with patients treated with direct microsurgical clipping of the aneurysm. This technique has become the preferred approach for microsurgical management even though there is persistent morbidity and the procedure is technically challenging. In fact, the rate of intraoperative rupture during clipping and wrapping of BAs is reported to be as high as 50%, probably contributing to poor outcomes.7 ,13
Similarly, conventional endovascular approaches including coil embolization, stent-assisted or balloon-assisted coil embolization, and vessel deconstruction with or without high-flow bypass have been associated with procedural morbidity and devastating clinical outcomes in some series,5 ,14 although others have reported favorable results with stent-assisted coiling and more recently layered-stent constructs with or without adjunctive coil use.15 ,16
Flow diversion represents a revolutionary approach to the treatment of cerebral aneurysms. Through the disruption of the pulsatile flow into the aneurysmal sac, flow diversion leads to stasis, thrombosis, and regression of the aneurysm itself.8 Histological studies following FD deployment revealed endothelialization of the device and reconstruction of the entire length of the parent vessel covered by the implant and across the aneurysmal neck. Flow diversion represents a paradigm shift, focusing on reconstructing the parent vessel rather than treatment of the aneurysmal sac. The first FD device approved by the FDA was the PED, which is a self-expanding FD made of a braided multi-alloy cylindrical mesh woven from platinum/8% tungsten and 35NLT (cobalt chromium nickel) alloy wires. The woven wires of the device provide nominally 30–35% metal coverage of the arterial wall surface area.
This represents a significant paradigm shift compared with the 6–8% metal surface area coverage currently present in devices used for stent-assisted coil embolization.17 Two clinical trials have been conducted in the USA: the Pipeline for the Intracranial Treatment of Aneurysms trial18 and the Pipeline for Uncoilable or Failed Aneurysms.19 The results of these trials led to the approval of PED by the FDA and the introduction of the device in clinical practice for the treatment of large, giant wide-necked, and complex unruptured proximal carotid aneurysms. Although, the FDA approved PED for the treatment of large unruptured saccular aneurysms of the ICA, subsequently the device has proven useful in treating smaller sidewall saccular aneurysms. However, all of these studies excluded patients with acute aSAH. Recently, several case series have demonstrated a possible role for FDs as a viable alternative to microsurgical approaches with acceptable rates of lesion occlusion and procedural morbidity/mortality in patients presenting with aSAH.9 ,20–26
Implanting a FD or a stent in acutely ruptured aneurysms has per se the risk of severe complications because of the need for dual antiplatelet therapy. A dual antiplatelet regimen is needed to avoid complications within the PED itself and distally such as in-stent thrombosis and possible thromboembolism. In addition, patients with aSAH may need surgical procedures such as extraventricular drain placement or replacement or delayed surgery for a ventriculoperitoneal shunt. In addition, because of the possible systemic complications of SAH, these patients may need gastrostomy, tracheostomy, decompressive craniectomy or other procedures. In this regard, there is no level 1A evidence for the use or the timing or of a particular antiplatelet regimen of a specific type nor for the initiation and duration of antiplatelet therapy.
In our series, the timing and type of the dual antiplatelet regimen was left to the best judgment of the operators at each institution. In particular, the dual antiplatelet regimen consisted of a 600 mg load of clopidogrel and 325 mg of aspirin 5 followed by daily doses of clopidogrel 75 mg and aspirin 325. At Baptist Hospital in Miami the treatment was started after placement of the EVD and continued for at least 6 months. At Gates Vascular Institute and SUNY SUNY University at Buffalo antiplatelet medication was started 12 h after insertion of the EVD with CT confirmation of no EVD-related hemorrhage.
Aspirin and clopidogrel were the most common agents used, but prasugrel and ticagrelor have also been used instead of clopidogrel secondary to a high rate of clopidogrel resistance. Confirmation of effective antiplatelet activity with VerifyNow was also obtained. Glycoprotein IIb/IIIa inhibitors were avoided. Dual antiplatelet coverage was maintained for at least for 3 months and in some cases, based on individual physician preference, for 6 months.
With regard to the use of PED in BAs overall, Yoon et al9 reported the largest series of ruptured BAs of the ICA treated with PED. The authors reported 12 ICA ruptured BAs in 11 patients treated at six institutions in the USA between May 2011 and March 2013. Nine of these patients (75%) received a single PED, one received two PEDs, one was treated with coils and one PED, and one was treated with coils and two PEDs. Of the 11 patients, three (27%) had major complications such as MCA territory infarct, vision loss, and death. The 10 patients who survived had good clinical outcomes (mRS score 0–2). On long-term follow-up angiography the aneurysm was completely obliterated in seven patients. In another case series by Çinar et al23 the authors reported a series of seven BAs all located in the supraclinoid ICA. In their series, flow diversion with PED was carried out in all but one patient who was treated by parent artery occlusion. Aspirin and clopidogrel were administered 6–8 h before treatment in all patients. No serious adverse events such as rebleeding or thromboembolic complications were reported. The clinical outcome was mRS ≤2 in five patients, one patient had mRS 3, and one had mRS 4. On long-term control angiography, all aneurysms were occluded without recanalization.
In our series, all patients presenting with SAH were reviewed and those with ruptured BAs were assessed retrospectively with respect to procedural technique and outcomes. All PED implants were performed successfully. All patients were treated with PED alone, without coils. There were no cases of parent artery or branch occlusion following PED implant and there were no serious periprocedural complications related to PED placement such as immediate or delayed hemorrhages or in-stent thrombosis. None of the patients in our series had thromboembolic complications. Clinical outcomes were excellent with 90-day mRS scores of 0 or 1 in nine patients; one patient died as a consequence of a large SAH (H&H grade 4). Long-term follow-up by DSA showed complete occlusion of all aneurysms with remodeling of the artery. There were no instances of recurrence, recanalization, or regrowth of any of the aneurysms.
Limitations of the study
Our retrospective case series is inherently limited in its design and by its relatively small sample size. Because aSAH secondary to ruptured BA is rare, enrolling patients in a randomized prospective study may be challenging. Multicenter prospective collection of data may prove beneficial in assessing outcomes in a larger series of patients. Such collection of data may provide a better level of evidence, particularly with regard to a standardized consensus-driven management of dual antiplatelet regimen before FD implantation.
Conclusion
The present series suggests that repair of ruptured BAs with PED alone may be a safe and durable option.
References
Footnotes
Contributors All listed authors have contributed to the conception, design, data acquisition and analysis, drafting of the manuscript and/or review and critical editing prior to submission.
Competing interests IL, GD and ASi are consultants for Medtronic. IL is a consultant for Stryker. GD is a consultant for Microvention. AS, IL and GD have received research grants from the National Institutes of Health. AS holds financial interests in Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, and Lazarus Effect and serves as a consultant to Blockade Medical, Codman & Shurtleff, Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, Lazarus Effect, MicroVention, Penumbra, Stryker Neurovascular, and Pulsar Vascular.
Ethics approval Ethics approval was obtained from the Review Boards of Baptist Health Miami and Buffalo University NY.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Additional unpublished data may be available on request by contacting the corresponding author via email.