Article Text
Abstract
Major ischemic strokes secondary to proximal artery occlusions are responsible for significant morbidity and mortality. Owing to extensive clot burden, these strokes are poorly responsive to intravenous tissue plasminogen activator. The introduction of endovascular therapy, particularly mechanical devices, has resulted in markedly improved recanalization rates of large vessel occlusions. With increasing experience with the Penumbra Stroke System and the 054 reperfusion catheter, there has been further improvement in TIMI 2 and 3 revascularization rates with faster times to vessel opening. The aim of this technical review is to convey various tips and tricks learnt from this experience.
- Catheter
- balloon
- thrombolysis
- stroke
- stent
- stenosis
- intervention
- hemorrhage
- embolic
- coil
- brain
- atherosclerosis
- angiography
- aneurysm
- aneurysm
- angioplasty
- arteriovenous malformation
- flow diverter
- thrombectomy
- artery
- MRI
- CT
- vein
- thrombectomy
- technique
- complication
Statistics from Altmetric.com
- Catheter
- balloon
- thrombolysis
- stroke
- stent
- stenosis
- intervention
- hemorrhage
- embolic
- coil
- brain
- atherosclerosis
- angiography
- aneurysm
- aneurysm
- angioplasty
- arteriovenous malformation
- flow diverter
- thrombectomy
- artery
- MRI
- CT
- vein
- thrombectomy
- technique
- complication
Introduction
Since its approval in 1995, intravenous recombinant tissue plasminogen activator (r-tPA) remains the only FDA-approved treatment of acute ischemic stroke. Owing in part to the restrictive time window after symptom onset, only a small percentage (1.8–4.3%) of all patients with ischemic stroke receive intravenous rt-PA therapy.1–3 Furthermore, it has been demonstrated to be less effective in large vessel occlusions.4
Intra-arterial (IA) revascularization approaches have emerged as an important therapeutic option more than 3–4.5 h after stroke onset. Local thrombolytic administration within the clot has been performed off-label since the PROACT II study demonstrated improved recanalization rates and patient outcomes compared with placebo, with an acceptable complication rate.5 These findings were confirmed in a subsequent meta-analysis.6
More recently, IA mechanical devices have been shown to revascularize proximal artery occlusions safely up to 8 h after stroke onset. These devices appear to further improve recanalization rates7 and offer another option to those patients who are ineligible or refractory to r-tPA. In 2004 the Merci Retriever (Concentric Medical, Mountain View, California, USA) became the first mechanical thrombectomy device cleared for human use in the USA. Revascularization rates in the Merci studies range from 43% to 55% (up to 68% with adjunctive therapy), with good functional outcomes (defined by mRS ≤2 at 90 days) reported in up to 36% of patients.8–10 Recanalization rates with the Merci system have generally increased since the initial trial owing to the introduction of more effective device designs and increased operator experience.
In 2008 the Penumbra System (Penumbra Inc, Alameda, California, USA), which uses continuous thrombus aspiration technology, was introduced in the USA. The Penumbra Pivotal Trial reported an 82% revascularization rate resulting in FDA 510(k) clearance.11 However, despite meeting its primary endpoint, this study was criticized for the low rate of 90-day functional independence (25%). Goyal et al provided an explanation for the conundrum posed by the high revascularization rate but lower than expected good outcome rate in the Pivotal Trial.12 Using the Alberta Stroke Program Early CT Score (ASPECTS) to analyze pretreatment CT scans from the Pivotal Trial, the Calgary investigators showed that patients with small infarcts at baseline (ASPECTS score >7) were more likely to achieve functional independence following revascularization with the Penumbra System than those with large infarcts at baseline (ASPECTS score ≤7) (50% vs 15%; p=0.0001). A total of 64% of patients in the trial presented with large infarcts at baseline according to the Calgary analysis. While it is not the intended topic of this paper, several authors strongly believe that this finding, along with other emerging data, support the use of advanced imaging selection to guide the use of IA therapy more appropriately.13 Subsequently, seven international centers published their collective post-market experience in the Penumbra POST trial, which reported an 87% revascularization rate and 41% rate of good functional outcome.14
Introduction of the Penumbra 054 System
The 054 catheter, which was added to the existing three catheters of the Penumbra System in 2009, features a larger 0.064 inch proximal internal diameter (ID) for enhanced aspiration efficiency. The recent Penumbra SPEED study assessed the safety and efficiency of the 054 catheter.15 Interim results reported a median aspiration time of 18 min (n=73) with the 054 System compared with 45 min using the three original catheters only (Penumbra Pivotal, n=125; p<0.001). The time from puncture to final revascularization similarly decreased to 53.5 min in the SPEED study from 97 min in the Pivotal Trial. The 054 catheter also demonstrated a high revascularization rate (TIMI 2–3) of 92% when used as frontline therapy prior to any adjunctive treatments.
While the importance of rapid revascularization is generally accepted, it must be acknowledged that none of the mechanical devices has demonstrated clinical efficacy over medical management alone. With this caveat in mind, the objective of this paper is to provide an overview of the techniques that have been developed to optimize the success of the Penumbra System in general and the new 054 catheter in particular.
Coaxial technique for the 054 System
The Penumbra System consists of four sizes of reperfusion catheters and separators identified by their distal IDs: 0.054 inch, 0.041 inch, 0.032 inch and 0.026 inch. Because the efficiency of aspiration increases to the fourth power of the catheter ID, it should be initiated with the largest reperfusion catheter allowed by the target vessel size in order to achieve rapid revascularization. The larger 0.054 inch and 0.041 inch systems are typically the starting catheters for internal carotid artery (ICA)/middle cerebral artery M1 and vertebral/basilar artery occlusions, respectively (figure 1).
Reperfusion catheter/separator sizing table. Vessel sizing may vary from patient to patient. Image courtesy of Penumbra Inc.
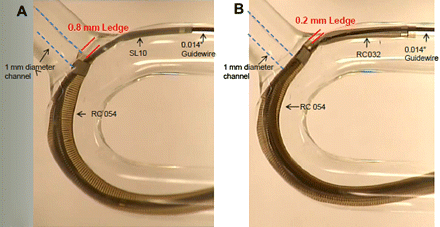
Because of its size, the 054 catheter requires the use of a coaxial technique to facilitate navigation to the site of occlusion. Figure 2 illustrates the ‘ledge effect’ when delivering the 054 reperfusion catheter over a 0.014 inch microwire alone. The size difference between the 0.054 inch ID and the 0.014 inch wire creates a ledge that may catch at vessel origins, such as the ophthalmic artery takeoff or the origin of the anterior cerebral artery A1 segment. To overcome this challenge, access with the 054 catheter and, to a lesser extent, the 041 catheter is optimized with a coaxial technique (figure 3). The smaller 032 and 026 reperfusion catheters can be delivered simply over either a 0.014 inch or 0.016 inch wire.
Ophthalmic glass model with 054 reperfusion catheter over 0.014 inch wire. (A) The ‘ledge effect’ with the 054 catheter tip may interfere with distal access and (B) is not resolved with a 0.014 inch guidewire. Image courtesy of Penumbra Inc.
Coaxial technique reduces ‘ledge effect’. (A) The SL10 microcatheter (Boston Scientific, Fremont, California, USA) reduces but does not eliminate the ledge created by the 054 catheter. (B) The 3.4 F distal outer diameter of the 032 reperfusion catheter results in a 0.2 mm ledge which facilitates tracking around the ophthalmic bend. Image courtesy of Penumbra Inc.
Set-up
The optimal coaxial set-up for the 054 reperfusion catheter consists of a 0.014 inch or 0.016 inch microwire inside the 032 reperfusion catheter over which the 054 reperfusion catheter is advanced. A long carotid sheath is recommended (figure 4).
Coaxial set-up for 054 catheter. Image courtesy of Penumbra Inc.
The Fathom 16 wire (Boston Scientific, Fremont, California, USA) offers advantages related to its size and torqueability. The increased stiffness of a 0.016 inch wire can provide more support and slightly straighten the curve of the vascular loop, thereby facilitating access (figure 5). However, a 0.014 inch wire of the operator's choice will also work.
Use of 0.018 inch wire for additional support. (A) The tip of the empty 054 catheter resides at the petrous-lacerum segment of the left internal carotid artery. (B) Insertion of the 032 catheter over the 0.018 inch Roadrunner Extra-Support microwire into the 054 catheter results in straightening of the cervical internal carotid artery. (C) The 054 catheter is advanced over the 032 catheter/Roadrunner system which has been placed into the M1 segment. (D, E) Pre- and post-aspiration angiograms showing recanalization.
The 032 reperfusion catheter best matches the size of the 054 reperfusion catheter to reduce the ‘ledge effect’. An additional advantage is that, following proximal thrombus aspiration, the 032 catheter may be reinserted through the 054 catheter to aspirate any remaining distal occlusions. Typically, the 032 reperfusion catheter is used in the M2 segment. Given the significant extra cost of the 032 catheter, other standard microcatheters such as the Renegade 18 (Stryker, Fremont, California, USA) and Rebar 18 (Covidien/EV3, Irvine, California, USA) can also be used as the inner coaxial catheter, but do not have aspiration capability. Table 1 lists the coaxial product compatibility of the individual Penumbra reperfusion catheters.
Coaxial product compatibility
In cases where additional support is needed to advance the 054 reperfusion catheter over the coaxial system, the use of a 0.018 inch wire such as the Roadrunner Extra-Support microwire (Cook, Bloomington, Indiana, USA) can be considered (figure 5). The Roadrunner Extra-Support wire consists of a nitinol alloy mandril and distal platinum spring tip. It is compatible with the RapidTransit (J&J Codman, Raynham, Massachusetts, USA), Prowler Select Plus and Renegade HI-FLO (Boston Scientific) microcatheters. Once the inner coaxial catheter is navigated over a 0.014 inch/0.016 inch microwire, the wire is exchanged for a Roadrunner 0.018 inch wire. Preferably, this wire should not be advanced out of the inner catheter. However, if this is necessary, the wire has a 5 cm very flexible tip.
Frequently, a 90 cm length 6 French (F) Shuttle Select Guiding Sheath (Cook) is used for access from the groin to the ICA. However, the tight fit between the 6 F Shuttle (0.087 inch ID) and the 054 reperfusion catheter (0.080 inch outer diameter, OD) produces a suboptimal (although adequate) angiographic contrast injection. For this reason, a 7 F long sheath may be a better choice. Additionally, in cases of significant carotid tortuosity, the 90 cm length may not allow the 054 reperfusion catheter to navigate far enough beyond the sheath to reach the full extent of the occlusion, in which case an 80 cm length sheath is preferred. Other available long sheaths include the Arrow-Flex Sheath (Arrow, Reading, Pennsylvania, USA) and the Pinnacle Destination Guiding Sheath (Terumo, Tokyo, Japan). Some of the authors have used the Merci 9 F Balloon Guide Catheter (Concentric Medical) to achieve proximal flow arrest or reduction during thromboaspiration of terminal ICA occlusions, thereby preventing distal ACA embolization. However, it has been suggested that such flow reduction may limit aspiration efficiency. The 9 F balloon guide catheter functions similarly to a 6 F shuttle sheath in terms of ability to perform angiography. The 80 cm length is preferred over the 95 cm length because it allows for greater distal access of the reperfusion catheter.
The coaxial set-up and navigation technique are similar for the 041 system. While a long carotid sheath can be used with the 041 system, it is not necessary. Most physicians use a regular 6 F sheath and a 90 cm length 6 F guiding catheter with a 0.070 inch lumen. The inner microcatheter must be at least 150 cm long with a proximal OD smaller than 2.8 F (table 1).
Access tips
When using a coaxial technique, a rotating hemostatic valve (RHV) should not be attached to the catheter system being targeted for aspiration. The lack of an RHV on the 054 catheter hub allows the 032 catheter to travel further past the tip of the 054 catheter (by the length of the RHV) for greater distal 032 catheter purchase. The long sheath is typically placed in the distal common carotid artery or proximal ICA. For maximum purchase, the wire can be placed in the M2 or M3 segment and the 032 reperfusion catheter in the M1 or M2 segment before navigating the 054 reperfusion catheter around the ophthalmic bend. The typical push/pull type motion of a coaxial system will facilitate catheter advancement. In difficult situations where a coaxial approach fails, alternative techniques have been employed to gain distal 054 catheter access (figures 6 and 7).
Use of Hyperglide balloon microcatheter to facilitate 054 catheter advancement. (A) Pretreatment angiogram showing a right M1 occlusion. (B) A Hyperglide 4 × 20 mm balloon (Covidien/EV3) is placed through the 054 catheter so that it extends approximately 5 mm out of the reperfusion catheter tip. Inflation of the balloon to the internal diameter of the 054 catheter removes the ‘ledge effect’ and allows advancement of the system over the Xpedion 10 (Covidien/EV3) microwire. (C) Post-aspiration angiogram showing revascularization.
Merci Retriever adjunct. The top two diagrams illustrate the typical coaxial approach in which the 054 catheter travels along the outer curve where it encounters the origin of the ophthalmic artery. Another technique to improve the trackability of the 054 reperfusion catheter is to change the angle with which the catheter navigates the ophthalmic segment and M1 origin. This may be performed by deploying an appropriately-sized Merci Retriever (Concentric Medical), such as a V2.0 or V2.5 soft, in the mid M1 segment through either the 032 catheter or an 18L (Concentric Medical) microcatheter. By applying gentle traction on the Merci Retriever, the course of the wire straightens, approximating the inner curve of the vasculature, pulling the catheter complex away from the ledge of the vessel origins (bottom left diagram). The 054 catheter can then be more readily advanced into the target vessel. Once the 054 reperfusion catheter is in place, the retriever is resheathed into the 18L and then removed prior to separator placement and aspiration (technique in press, unpublished). Image courtesy of Cleveland Clinic Foundation.
Once the 054 reperfusion catheter is delivered to the occlusion site, the 032 catheter and guidewire are removed and an RHV with aspiration tubing is attached to the 054 reperfusion catheter. A 054 separator is then navigated to the tip of the catheter, the aspiration pump is turned on and thrombus removal is initiated.
Observing the blood flow in the aspiration tubing and canister
The aspiration pump should be tested prior to use in patients by placing the aspiration tubing into a bowl of saline and turning on the pump. It is important to ensure that the aspiration gauge reads −20 inch Hg. During treatment the flow of blood within the aspiration tubing and canister provides the best feedback on the progress of thromboaspiration. Before engaging the thrombus, the reperfusion catheter should be positioned several millimeters proximal to the proximal aspect of the thrombus and aspiration should be performed to observe the flow of blood in the aspiration tubing and canister. The flow should be constant with a reasonably high rate (depending on the catheter size used). This is the baseline flow rate. If adjunctive intra-arterial tPA is being used, this can be administered through the aspiration catheter at the thrombus interface prior to aspiration. Once the reperfusion catheter is placed within the clot, the flow should slow or even stop. This is a positive sign that the reperfusion catheter has engaged the thrombus and is now appropriately clogged. To restore flow, the separator should be repeatedly manipulated in and out of the reperfusion catheter to clear the clot.
Manipulation of the separator should continue until flow in the aspiration tubing is restored to baseline. The reperfusion catheter is then advanced forward until flow again slows or stops, followed by another round of separator manipulation. A good practice is to repeat these steps for a few minutes followed by a contrast run through the guiding catheter to check progress. Injections should not be made through the reperfusion catheter as there may be residual clot within the lumen. These steps should be repeated until antegrade blood flow is restored. If progress is slow or difficult, troubleshooting steps (outlined below) should be undertaken.
After flow is restored, a control angiogram should be performed to check for any remaining distal thrombus that is amenable to further aspiration. If present, the reperfusion catheter and separator should be downsized as appropriate for the more distal vessel being treated.
Manipulating the separator
The separator is designed to clear the reperfusion catheter lumen so that continuous aspiration can be maintained. The separator is constructed of a solid piece of wire that is ground to various diameters. The distal end consists of a polymer bulb followed by a 6 mm length tip that has a platinum coil overwind for added flexibility and softness as well as for improved fluoroscopic visualization. The distal tip is designed to be similar in construction and softness to a soft neuro guidewire and is thus similarly atraumatic. The purpose of the polymer bulb is to macerate clot inside the reperfusion catheter. The bulb is sized for adequate clearance within the catheter to maintain retrograde flow (ie, aspiration). There is a gold marker band inside the polymer bulb to enhance visualization (figure 8). The separator is not a torqueable or steerable component and should be switched out for a guidewire if navigation is required for a more distal occlusion.
Visualization of separator markers. Image courtesy of Penumbra Inc.
When manipulating the separator, the separator bulb marker should completely exit the reperfusion catheter and then be withdrawn completely inside the catheter. A useful guide is to bring the separator out so that there is a distance of 4 mm between the separator bulb marker and the reperfusion catheter marker. The separator should be withdrawn 20 mm inside the reperfusion catheter (figure 9).
Separator movement. During manipulation the separator should completely exit the catheter and be withdrawn completely into the catheter. Separator excursion is gauged using the separator bulb marker which should extend 4 mm from the reperfusion catheter tip and should be withdrawn 20 mm into the catheter. Image courtesy of Penumbra Inc.
It is important not to extend the separator too far beyond the reperfusion catheter tip. The vessel anatomy can be difficult to visualize when working inside the clot. To ensure the proper ‘throw’ distance, the exiting distance of the separator should be checked at the beginning of the procedure and the torque device set on the separator wire accordingly.
When the separator is engaged in the clot there will be some resistance to separator manipulation. While this is normal, attention should be paid to how the separator tip appears on fluoroscopy. If resistance increases and the tip appears to be deformed, the separator should be exchanged for a new one.
Aspirating from distal to proximal at bifurcations
Proximal to distal aspiration works well in the majority of cases. The main advantage of this method is the ability to assess the progress of clot aspiration using blood flow in the tubing and via contrast injections from the guiding catheter. However, when the clot arises at a bifurcation, it may be beneficial to aspirate distally first and then to work proximally. If the occluded branch has an acute takeoff angle it may be difficult to achieve a stable position of the reperfusion catheter within the proximal clot face. Consequently, separator manipulation at a hard clot may push the reperfusion catheter out of the occluded branch. The distal to proximal aspiration approach can be helpful in these situations.
For this approach, the occluded branch should be selected with a microwire and the inner coaxial microcatheter advanced over it and through the clot. Next, an angiographic road map should be performed through the inner microcatheter to ensure an intravascular position and to guide distal placement of the wire and microcatheter for safe advancement of the reperfusion catheter. The road map is also critical for thromboaspiration because it outlines the distal extent of the clot and the adjacent vascular anatomy for safe manipulation of the separator. A dual contrast injection from the guide catheter at the proximal end and the inner coaxial microcatheter at the distal end may be performed to demonstrate both the proximal and distal anatomy.
After identifying the distal end of the thrombus, the reperfusion catheter should be advanced over the inner coaxial microcatheter to the end of the thrombus. Aspiration and separator manipulation can then start and proceed proximally. Also, aspiration may be performed as the reperfusion catheter is being advanced through the thrombus to minimize downstream emboli.
An alternative approach when navigation into one branch is difficult is to use a standard microwire in place of a separator during thromboaspiration. In this way, if the reperfusion catheter is pushed out of the occluded branch, access can be regained over the microwire. This approach helps to clear the thrombus away from the bifurcation where standard techniques can then be employed.
Troubleshooting
In cases involving extremely high clot burden or very fibrous clot, flow can be difficult to restore even after a few rounds of separator manipulation. If several minutes have elapsed without blood flow within the aspiration tubing, it is necessary to clear the system. Occasionally there is enough clot on the separator bulb or at the tip of the reperfusion catheter that additional steps are required to clear the catheter.
The troubleshooting steps outlined below should be attempted sequentially, proceeding to the next if the previous one fails:
Bring the reperfusion catheter and separator to a patent segment of the vessel and manipulate the separator. The increased retrograde flow from aspiration may help to dislodge the adherent material.
Remove the separator and wipe down any adherent material. If using the 054 system, it may be sufficient to withdraw the separator to the proximal part of the catheter where the lumen may be large enough to allow thrombus removal by aspiration. It is important always to maintain continuous aspiration when pulling back or removing the separator.
Remove the reperfusion catheter and separator and flush outside of the body. Replace any damaged components.
It is also important to make sure that the aspiration tubing itself is not obstructed with clotted blood by disconnecting it from the catheter and flushing it with saline.
Make sure that the aspiration pump is operating at the appropriate vacuum setting (−20 inch Hg).
Conclusion
Neurointerventionalists have increasingly powerful mechanical tools for achieving revascularization in acute ischemic stroke. As with any new procedure or device, operator experience is critical for both safety and success. To this end, this paper highlights techniques learned from experience that will facilitate use of the Penumbra System.
References
Footnotes
Competing interests AJY receives research support from Penumbra Inc for the Core Imaging Lab for the START trial. DF is a consultant and/or serves on the speakers' bureau for Penumbra, Stryker Neurovascular, Genentech and Codman. AST and DVH are consultants for Penumbra Inc. JAH is a shareholder in Intratech.
Provenance and peer review Not commissioned; externally peer reviewed.