Article Text
Abstract
Background and objective Flow diverters are an exciting new class of endovascular devices that treat aneurysms by curative reconstruction of the parent artery. The Pipeline embolization device (PED) is the first FDA-approved intracranial flow diverting device available in the USA. This paper presents periprocedural results with the device in a series of 35 consecutive cases.
Methods All patients who underwent PED treatment of an intracranial aneurysm at our institution following FDA approval of the device in April 2011 were included in the series. Patient demographics, aneurysm characteristics, procedural details and technical and clinical outcomes were analyzed.
Results Thirty-four patients (age range 23–78 years, mean 56.4 years) with 41 unruptured aneurysms (37 anterior circulation, four posterior circulation, mean size 11.4 mm, 20/21 large or giant) were treated with the PED in 35 cases (one patient had bilateral aneurysms treated on 2 separate occasions). Thirty-four of 35 cases (97%) were successfully completed. A total of 64 PEDs were implanted, with a mean number of 1.2 PEDs implanted per anterior circulation cases and 6.5 per posterior circulation cases. A single PED was implanted in 73% of cases. Immediate flow disruption occurred in 97% of the cases. The overall rate of major stroke or mortality was 3% (1/35 patients). Minor stroke, cranial nerve palsy, transient neurological deficit and groin complication occurred in one patient each (3% each, 12% total).
Conclusion Treatment of cerebral aneurysms with the PED carries an acceptable risk profile when a rigorous and uniform technique is used. Although the long-term results will need to be analyzed, the immediate procedural outcomes in the study series using this technique appear quite promising.
- Aneurysm
- endovascular
- flow diversion
- pipeline embolization device
- arteriovenous malformation
- coil
- flow diverter
- liquid embolic material
- arteriovenous malformation
- angiography
Statistics from Altmetric.com
- Aneurysm
- endovascular
- flow diversion
- pipeline embolization device
- arteriovenous malformation
- coil
- flow diverter
- liquid embolic material
- arteriovenous malformation
- angiography
Introduction
Endovascular treatment of intracranial aneurysms has recently focused on an endosaccular approach to aneurysm obliteration. Following the introduction of Guglielmi detachable coils two decades ago, advances in coil technology and the use of adjunct devices, including stents and microballoons, have facilitated treatment and improved outcomes for endosaccular aneurysm embolization.1–9 However, significant challenges still remain in the use of these techniques for treatment of larger and fusiform aneurysms, with recanalization rates of up to 50–60%.10–12
Flow diverters represent a novel class of endoluminal devices that promote parent vessel reconstruction, a strategy ideally suited for aneurysms with diffuse circumferential involvement of the parent vessel. The Pipeline embolization device (PED; Covidien Vascular Therapies, Mansfield, Massachusetts, USA) is a low-porosity ‘stent-like’ cylindrical device consisting of 48 individual strands of platinum and cobalt chromium. This mesh provides 30–35% metal surface area coverage when fully deployed compared with 6.5–9% for conventional self-expanding intracranial stents/vascular reconstruction devices.13 The PED has been available internationally for several years, and initial experiences with the device have primarily come from these large single-center international groups and multicenter registries.14–17 Additional early experience with the device came from groups in the USA using it on a compassionate basis as well as those groups participating in a multicenter trial for FDA approval.13 ,18 ,19 Based on these case reports and series, endovascular reconstruction using the PED has been shown to be an effective, durable and safe treatment. Aneurysm occlusion rates of approximately 90% were achieved at 6 months.14 ,16 Significant morbidity and mortality rates of 0–6.8% and minor complication rates of 11% have been reported.14 ,16 ,20 Results from the Pipeline for Uncoilable or Failed Aneurysms (PUFS) trial, which included eight investigational sites in the USA and two international sites, demonstrated that stroke occurred in 8/107 patients (7.5%) and major ipsilateral stroke or death occurred in 6/107 patients (5.6%).19
Based on data from the PUFS trial as well as other clinical series, the PED was recently approved by the FDA for use in the USA. The PED has been used at our institution only following FDA approval. We present a single-center, single-user, all-inclusive series of periprocedural outcomes for 35 cases of aneurysm embolization with the PED.
Methods
Patient selection
We retrospectively reviewed the records of a prospective single-center aneurysm database to identify all patients who underwent endovascular aneurysm treatment using the PED since FDA approval of the device in the USA on 6 April 2011. Data were collected prospectively with respect to patient demographics, aneurysm size and morphology, details of the interventional procedure, anticoagulation regimens and technical and clinical outcomes. All patients presented with unruptured aneurysms. Patients presenting with severe headache and clinical suspicion for subarachnoid hemorrhage (SAH) were screened with a CT scan of the head and lumbar puncture to rule out a hemorrhage event.
Endovascular procedure
Embolization procedures were performed on a biplanar flat panel angiographic system (Artis zee, Seimens, Erlangen, Germany) under general anesthesia. All patients were treated preoperatively with a dual antiplatelet regimen consisting of aspirin 325 mg daily and clopidogrel 75 mg daily for 7 days prior to the intervention. Platelet inhibition assays were not routinely performed. All procedures were performed with systemic anticoagulation using heparin. The anticoagulation regimen consisted of a bolus of 5000 units administered at the start of each case followed by an intra-procedure re-bolus of 1000 units each hour.
Accurate vessel size measurements for the parent vessel were determined from calibrated standard digital subtraction angiogram (DSA) images either at the time of the intervention or from the preceding diagnostic angiogram. The goal of each procedure was complete coverage of the aneurysm neck or fusiform/diseased segment of the vessel with a PED. When necessary, multiple PEDs were deployed in a telescoping fashion to achieve this goal. For all anterior circulation aneurysms a triaxial system was used through femoral access. This consisted of a Flexor Shuttle sheath (Cook Medical, Bloomington, Indiana, USA), a guide catheter and a Marksman microcatheter (Covidien Vascular Therapies). Guide catheters used were the Neuron (Penumbra, Alameda, California, USA), the DAC (Concentric Medical, Mountain View, California, USA) and the ReFlex Intracranial Catheter (Reverse Medical Corporation, Irvine, California, USA). For one of the posterior circulation aneurysms a biaxial system was used through brachial access. This biaxial system consisted of a ReFlex Intracranial Catheter as the guide catheter and a Marksman microcatheter. PEDs were deployed under real-time visualization using a combination of native fluoroscopy, road-map and DSA. For anterior circulation aneurysms, the distal PED was opened in the ipsilateral supraclinoid internal carotid artery (ICA) or, more commonly, in the ipsilateral M1 segment. For posterior circulation aneurysms, the distal PED was opened in the mid to distal basilar artery or a P1 segment. Proper device expansion and deployment was assessed with native fluoroscopy and DynaCT. If a partially deployed PED did not open properly secondary to device failure or was malpositioned, the device was removed. Balloon angioplasty was used in one case in order to dilate a short segment of the PED that did not fully expand.
If significant vasospasm was encountered during the procedure, intra-arterial nicardipine was administered as an antispasmodic agent. Nicardipine was given either directly via hand injection through a microcatheter or it was added to the heparinized saline flush bag (final concentration 1 mg/1000 ml). If intra- or post-procedure thrombus was present in the PED, a bolus of abciximab (Eli Lilly, Indianapolis, Indiana, USA) was administered intra-arterially (3–5 mg over approximately 15 min) for acute thrombolysis followed by an intravenous abciximab infusion (0.125 μg/kg/min) over 24 h. Patients treated with abciximab were also anticoagulated with systemic intravenous heparin (goal activated partial thromboplastin time (aPTT) ratio 2.0–2.5) for 48 h after the procedure. Post-procedure systemic heparinization was not routinely used for anterior circulation cases unless the patient was treated with abciximab. For posterior circulation cases, which typically involve longer PED constructs, some patients were treated with 12–48 h of post-procedure systemic anticoagulation with heparin (goal aPTT ratio 2.0–2.5). Dexamethasone 10 mg was routinely given intravenously at the beginning of each case, followed by 4 mg every 6 h for 24 h after the procedure. Additionally, a single dose of intravenous antibiotics (typically cefazolin or clindamycin) was given prior to femoral puncture. This policy of administering prophylactic antibiotics before the procedure was started following a single groin site infection and it was followed in 28 of the 35 cases.
Control DSA was performed immediately after deployment and at 5 and 10 min after deployment to confirm patency of the parent vessels and to rule out intraluminal thrombus. Intra-deployment DSA runs were not routinely performed. At the end of the procedure, a DynaCT scan without contrast was performed to assess PED morphology and to evaluate for intracranial hemorrhage. When post-procedure systemic heparinization was not performed, the systemic heparin given during the procedure was allowed to wear off. Heparin reversal was not performed. Following sheath removal, hemostasis was obtained either with manual compression or an Angio-Seal closure device (St Jude Medical, St Paul, Minnesota, USA). After the procedure the patients were monitored overnight in a neurocritical care unit. Most patients were discharged home on the first postoperative day.
Data collection and statistical analysis
For demographics, the patient's age, sex and race were collected. Aneurysm characteristics assessed included aneurysm location (anterior circulation: supraclinoid ICA, paraophthalmic/clinoidal ICA, cavernous ICA, petrous ICA; posterior circulation: proximal/mid basilar, vertebrobasilar, posterior inferior cerebellar artery (PICA)), size (<10 mm, 10–20 mm, >20 mm), type (saccular, fusiform, dissecting) and aneurysm status (ruptured vs unruptured). The specifics of each procedure were also evaluated. These included the type of procedure access (triaxial vs biaxial), type of guide catheter (Neuron, DAC or ReFlex), length of the Marksman catheter (135 cm or 150 cm), fluoroscopy time, total intravenous heparin and use of an arterial closure device. The total number of PEDs used and the number of PEDs per case were also recorded. Additionally, cases with the adjunctive use of coils, intra-arterial nicardipine, balloon angioplasty, removal of an incompletely deployed PED and post-procedure systemic heparinization were assessed.
Immediate procedural outcomes were also evaluated. These included the number of cases successfully completed, length of post-procedure hospital stay, number of patients discharged home and immediate angiographic results. Immediate angiographic results were graded based on contrast stasis within the aneurysm. Mild stasis was defined as contrast visualized in the aneurysm until the late arterial phase; moderate stasis was defined as contrast visualized in the aneurysm until the capillary phase; pronounced stasis was defined as contrast visualized in the aneurysm until the venous phase; complete occlusion was defined as no contrast visualized in the aneurysm during a DSA run immediately after deployment.
Procedural and periprocedural complications were recorded and evaluated. These categories included mortality, transient neurological deficit, major and minor stroke, intracerebral hemorrhage (ICH), SAH, cranial nerve palsy, PED thrombosis, vessel dissection and groin complications. Major stroke was defined as stroke present after 7 days that increased the NIH stroke scale by ≥4 points, and minor stroke was defined as a stroke that resolved completely within 7 days or increased the NIH stroke scale by ≤3 points.19 Groin complications included groin infection or significant groin hematoma, defined as a hematoma requiring prolonged hospital stay or preventing ambulation.
Data are presented as counts, percentages and means. When means are presented, the SEM is used to assess sample distribution.
Results
Patient and aneurysm characteristics
Following FDA approval of the PED in April 2011, 34 patients (mean age 56.4 years, range 23–78) with 41 aneurysms were treated with the PED at our institution by the senior author (ALC) from 9 August 2011 to 27 December 2011. The characteristics of these patients and their aneurysms are presented in table 1. Of the 34 patients, 27 (79%) were women and seven (21%) were men. Twenty-three (67%) of the patients were white, eight (24%) were black, two (6%) were Asian and one (3%) was Hispanic.
Patient demographic data and aneurysm characteristics
Of the 41 treated aneurysms, 37 (90%) were located in the anterior circulation and four (10%) involved the posterior circulation. The majority of the anterior circulation aneurysms were located in the paraophthalmic/clinoidal ICA (23/41, 56%) and cavernous ICA (12/41, 29%), with the supraclinoid ICA and petrous ICA representing a total of 5% of the aneurysms. Four of the aneurysms in the series were located in the posterior circulation. The vertebrobasilar junction was involved in two cases (5% of total aneurysms) and the mid/proximal basilar and PICA each represented one of the treated aneurysms.
The mean size of the aneurysms treated was 11.4 mm. Using the size classification of the International Study of Unruptured Intracranial Aneurysms, 21 (51%) of the aneurysms treated were small (<10 mm), 18 (44%) were large (10–25 mm) and two (5%) were giant (>25 mm).21 Of the 41 treated aneurysms, 18 (44%) were saccular, 21 (51%) were fusiform and two (5%) were dissecting. Fusiform aneurysms were defined as involving >25% of the parent artery circumference. None of the patients treated in this series presented with SAH and none had pre-existing indwelling endoluminal devices.
PED treatment and procedure characteristics
Thirty-five treatments were performed in 34 patients with a total of 41 aneurysms. The details of these procedures, including PED use, are shown in table 2. One patient had bilateral paraophthalmic ICA aneurysms and these were treated on separate dates. Three patients had multiple aneurysms that were treated during a single procedure. For each of these three patients, the additional aneurysms were ipsilateral and adjacent to the primary aneurysm.
Procedural parameters
A femoral approach with a triaxial system was used for 34 (97%) of the 35 cases. For one case (3%), a brachial approach with a biaxial system was used to treat a vertebrobasilar aneurysm. The Neuron and ReFlex guide catheters were both used in 15 cases (43%) and the DAC was used in five (14%). The majority of Marksman microcatheters used were 150 cm in length (n=25, 71%) as this facilitated opening the PED in the M1 for anterior circulation cases. The fluoroscopy time for each case was used as a gauge of procedure length, and the mean fluoroscopy time for anterior circulation procedures was 45.0±3.7 min compared with 109.2±50.8 min for posterior circulation procedures. Mean intravenous heparin administered for each case—also a factor of procedure length—was also evaluated. In anterior circulation cases 5600±150 units of heparin were used compared with 11300±3600 units in posterior circulation cases.
A total of 69 PEDs were used in this series of 35 cases and 64 (93%) were successfully implanted. The five PEDs not implanted, representing 7% of all PEDs used, were removed following partial deployment and were each in a separate case. In three of these cases the PED was removed secondary to technical failure of the device to open and in two cases the PED was removed secondary to malposition. One of the procedures was aborted following removal of a partially deployed PED, without successful PED implantation in this patient. PED implantation was therefore successful in 34 of 35 cases (97%). Of the 34 cases with successful PED implantation, a single PED was placed in 25 cases (73%) and two PEDs were implanted in seven cases (21%). More than three PEDs were implanted in only two cases (6%), and both of these were for treatment of posterior circulation aneurysms. The mean number of PEDs implanted for anterior circulation aneurysms was 1.2±0.1 (range 1–2) and for a posterior circulation aneurysm it was 6.5±4.0 (range 1–18). In one patient, post-implantation balloon angioplasty was used for dilation of a focal segment of the PED.
Adjunctive coils were used in three cases (9%). Two of these cases were for anterior circulation aneurysms, and the coils were placed during the same procedure immediately prior to PED implantation. The third case was a patient with a traumatic dissecting PICA aneurysm that had a recurrence following coil embolization 18 days previously. Vasospasm requiring intra-arterial nicardipine treatment was encountered in three cases (9%). In two of these cases, the nicardipine was added to the guide catheter flush bag (final concentration 1 mg/1000 ml) and in one case the nicardipine was administered by a hand injection through the microcatheter.
Additionally, the Angio-Seal closure device was used for hemostasis in 10 (29%) of the 35 cases.
Immediate procedural outcomes and angiographic results
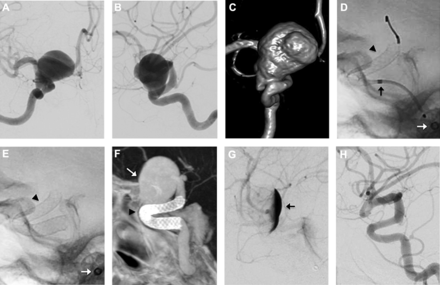
The immediate angiographic results and procedural outcomes are presented in table 3. As mentioned above, PED implantation was successful in 34 of the 35 cases (97%), representing successful completion of 30 of 31 anterior circulation cases and all four posterior circulation procedures. An example of a successful case is shown in figure 1, demonstrating treatment of a 21 mm paraophthalmic aneurysm with a single 4.0 mm × 16 mm PED. The one anterior circulation case that was not successfully completed was an 8 mm paraophthalmic aneurysm with extreme tortuosity of the supraclinoid ICA and the M1 segment. The distal PED did not fully expand in this situation and the procedure was aborted following removal of the partially deployed PED.
Immediate procedural outcomes
PED treatment of a 21 mm paraophthalmic aneurysm with complete occlusion at 3 months. (A, B) Pre-embolization digital subtraction angiogram (DSA) (A, transorbital oblique view; B, lateral view) and (C) 3D-rotational reconstructed images demonstrating the 21 mm paraophthalmic internal carotid artery aneurysm. (D, E) Native fluoroscopy during (D) and after (E) deployment of a 4.0 mm × 16 mm PED (arrowhead). In (D) the proximal PED is being deployed from the distal end of the Marksman catheter (black arrow). The distal end of the DAC guide catheter is also visualized (white arrow). (F) DynaCT angiogram demonstrating a fully expanded PED (arrowhead) with good vessel wall apposition. The aneurysm is marked with a white arrow. (G, H) DSA (lateral view) immediately after implantation (G) and at 3 months follow-up (H). (G) Immediately after implantation there is significant contrast stasis in the aneurysm that persists well into the late venous phase. This represents the ‘eclipse sign’ (arrow). (H) At 3 months follow-up there is complete occlusion of the aneurysm.
At the end of the procedure, complete angiographic occlusion of the aneurysm occurred in only one patient (3%), and this patient had a 6 mm fusiform cavernous aneurysm. Pronounced contrast stasis was observed in 22 patients (64%), with moderate stasis and mild stasis occurring in five patients each (15% each). No contrast stasis was observed in only one case (3%), and this patient had a 3 mm paraophthalmic aneurysm.
The mean length of post-procedure hospital stay for patients with anterior circulation aneurysms following PED treatment was 1.9±0.4 days. For posterior circulation aneurysms the mean length of stay was 14.7±7.4 days. Two of the patients with posterior circulation aneurysms had total hospital stays of >45 days. These prolonged stays were not related to PED treatment. One patient had a prolonged hospital course after presenting with a gunshot wound to the head, and his post-procedure hospital stay was 15 days. The other patient had unrelated significant gastrointestinal problems, and his hospital stay was 35 days after the procedure. In total, 31 of 35 patients (89%) were discharged home, 29/31 (94%) following treatment of anterior circulation aneurysms and 2/4 (50%) following treatment of posterior circulation aneurysms.
Procedural and periprocedural complications
Complications from the embolization procedures are presented in table 4. Severe procedural complications including SAH and subsequent death were encountered in one patient (3%). This patient had a single PED implanted across a 3 mm wide-necked paraophthalmic aneurysm (figure 2). The procedure was technically uneventful, without concern for wire perforation, and the patient awoke from anesthesia neurologically intact. DynaCT performed after PED deployment was negative for hemorrhage. Several hours afterwards, while being monitored in the neurocritical care unit, the patient had a change in mental status and a CT scan of the head showed an acute SAH (figure 2). Despite maximal surgical and medical interventions, this patient ultimately died secondary to complications from the SAH. An autopsy showed SAH from the known left paraophthalmic aneurysm. No other cerebral aneurysms were found.
Procedural and periprocedural complications
Aneurysm rupture following embolization of a 3 mm paraophthalmic aneurysm. (A) Pre-embolization digital subtraction angiogram (anterior-posterior view) and (B) 3D-rotational reconstructed images demonstrating the 3 mm paraophthalmic internal carotid artery aneurysm (arrow). (C) Native fluoroscopy and (D) DynaCT images, both oblique views, demonstrating the 4.0 mm × 16 mm PED status after successful deployment. The PED is fully open with good vessel wall apposition. (E) Immediate post-deployment control angiography (anterior-posterior view). The arrow labels the aneurysm. (F) CT scan of the head without contrast taken 5 h after the embolization procedure demonstrating diffuse subarachnoid hemorrhage. (G) Repeat angiography performed following the head CT scan demonstrating opacification of the aneurysm but no contrast extravasation.
Using the criteria from the PUFS trial, no patients in this series had a major stroke.19 Two patients (6%) had acute non-occlusive thrombus formation in the PED. For one of these patients, the thrombus was discovered at the end of the procedure during the final control angiography runs (figure 3). This patient was treated emergently with an intra-arterial ReoPro bolus followed by an intravenous ReoPro infusion for 24 h and intravenous systemic anticoagulation with heparin for 48 h. The patient remained neurologically intact throughout these events and the subsequent hospital course. For the second patient, contralateral hemiparesis developed 2 h after the procedure. Following a CT scan of the head which was negative for hemorrhage, an emergency angiogram demonstrated acute non-occlusive thrombus in the PED lumen. This patient was also treated with an intra-arterial ReoPro bolus followed by intravenous ReoPro infusion for 24 h and intravenous systemic anticoagulation with heparin for 48 h. Post-thrombolysis, the patient's contralateral hemiparesis resolved. However, ipsilateral leg weakness developed, which was attributed to an embolic event across the anterior communicating segment to the contralateral anterior cerebral artery territory. Despite no evidence of stroke on follow-up imaging, this leg weakness persisted until discharge to rehabilitation on 7 days after the procedure. This stroke increased the NIH stroke scale by 3 points and was classified as a minor stroke (3%).
PED treatment of a 13 mm paraophthalmic aneurysm complicated by intraluminal PED thrombosis. (A) Pre-embolization digital subtraction angiogram (DSA) (lateral view) and (B) 3D-rotational reconstructed images demonstrating the 13 mm paraophthalmic internal carotid artery aneurysm (arrow). (C) Native fluoroscopy and (D) DynaCT images, both oblique views, demonstrating the 4.0 mm × 16 mm PED after successful deployment (arrowhead shows distal end; arrow shows proximal end). Prior to deployment of this PED, a separate 4.0 mm × 14 mm PED had to be removed secondary to failed distal deployment. (E, F) Immediate post-deployment control angiogram (E, lateral view; F, anterior-posterior view) demonstrating near complete occlusion of the aneurysm and acute thrombus in the PED lumen (arrow). There is also decreased opacification of the anterior cerebral artery (arrowhead), likely secondary to thrombus. The arrow indicates the aneurysm. (G, H) Control angiogram (anterior-posterior view) DSA after 10 mg intra-arterial ReoPro (G) and 2 days of intravenous ReoPro infusion (F). After the initial intra-arterial ReoPro treatment there is decreased thrombus in the PED (arrow) and normal filling of the anterior cerebral artery (arrowhead). After the complete ReoPro treatment the internal carotid artery is widely patent with no residual thrombus (arrow). Also note complete opacification of the aneurysm.
Transient neurological deficit occurred in one patient (3%). This patient had a prior left-sided middle cerebral artery stroke and, following PED treatment of a left ICA aneurysm, the patient had transient mild speech and vision changes in the setting of elevated systolic blood pressure. Additionally, one patient (3%) had a cranial nerve palsy following PED treatment of a giant vertebrobasilar aneurysm. The patient developed a sixth nerve palsy attributed to thrombosis of the aneurysm. None of the patients in the series had an iatrogenic vessel dissection. However, one patient (3%) had a groin complication. Two weeks after the procedure, in which femoral access was used and hemostasis obtained with an Angio-Seal closure device, the patient developed cellulitis at the groin site. This was treated successfully with a short course of oral antibiotics.
Discussion
We report a single-institution, single-user series of 34 patients with 41 unruptured aneurysms that were treated with the PED following its FDA approval in the USA in April 2011. Ninety percent of the aneurysms were in the anterior circulation, with a similar proportion of aneurysms categorized as both small (<10 mm) and large/giant (>10 mm). Thirty-four of 35 procedures (97%) were successfully completed. A total of 64 PEDs were implanted, with a mean of 1.2 PEDs implanted per anterior circulation cases and 6.5 per posterior circulation cases. A single PED was implanted in 73% of cases. Immediate flow disruption, as evidence of contrast stasis in the treated aneurysms, was present in 97% of the cases performed. Eighty-nine percent of the patients were discharged home following the embolization procedure. Of 35 cases in this series, a severe complication of death secondary to SAH occurred in one patient (3%). Minor stroke, cranial nerve palsy, transient neurological deficit and groin complication occurred in one patient each (3% each). The overall rate of major stroke or mortality in this series was 3% (1/35 cases).
Aneurysm treatment with flow diverting devices is rapidly becoming a suitable and, in certain cases, preferred alternative to traditional endosaccular therapy with coils. Coil embolizaion is often criticized for high recurrence rates and incomplete aneurysm occlusion compared with microsurgical clipping, particularly in larger aneurysms and those with wide necks.12 ,22–24 The shortfall of endovascular coiling, despite advances in coil technology and the use of adjunctive stents and balloons, has been attributed to an inability to completely reconstruct the parent vessel at the aneurysm neck, limitations in coil packing density and failure to address the underlying diseased segment of parent vessel to prevent regional recurrence.10 Embolization with the PED, however, addresses these issues. The PED construct results in mechanical flow disruption followed by aneurysm thrombosis and ultimately parent vessel remodeling with endothelialization of the construct. When the aneurysm is completely excluded from the circulation, the thrombus resorbs and the aneurysm collapses around the construct.10
Various early published trials and case series of PED use, primarily from international groups, have demonstrated that the device is safe and effective for aneurysm treatment. The PUFS clinical study was a prospective single-arm multicenter trial that provided the primary evidence for safety and efficacy of PED use. Patients in this trial had large or giant aneurysms of the paraophthalmic, cavernous or petrous ICA with neck size ≥4 mm.19 Of the 108 patients treated, PED placement was successful in 107 patients (99%). A total of 341 devices were implanted, with a mean of 3.1 PEDs placed per aneurysm. A single PED was implanted in two of 107 patients (2%). Severe complications of major ipsilateral stroke or neurological death were reported in six of the 107 patients (5.6%). ICH occurred in five patients (4.7%), and four of those were prior to discharge from hospital. No SAH was reported. These results are similar to those in our series, in which PED placement was successful in 34 of 35 patients (97.1%) with severe complications occurring in one patient (3%). However, no patients in our series had an ICH compared with five patients in the PUFS trial. Additionally, a single PED was used in 73% of our cases compared with 2% in the PUFS trial.
The PED for the Intracranial Treatment of Aneurysms (PITA) trial was a multicenter single-arm non-randomized clinical trial conducted at three European centers and one center in Argentina in 31 patients with 31 aneurysms that were wide-necked or had failed previous endovascular treatment.17 In this trial, a total of 47 devices were placed with a mean of 1.52 devices per aneurysm. A single PED was used in 18 of the 31 cases (58.1%). PED placement was technically successful in 30 of the 31 aneurysms (96.8%). Severe complications of major stroke occurred in two patients (6.5%), with no reported minor strokes. The results of the PITA trial are also approximately in line with those from our series, except we reported a severe complication rate of 3% compared with 6.5% in the PITA trial.
Published reports from large series and registries show similar or, in some reports, better results. Lylyk and colleagues reported treatment of 53 patients with 63 aneurysms with the PED in Buenos Aires, although six of the patients were also included in the PITA trial.14 Seventy PEDs were implanted and no major complications were reported (0%). Minor complications did occur in six of the 53 patients (11%), including exacerbation of cranial neuropathy, groin hematoma and rash. Szikora and colleagues reported PED treatment in 18 patients with 19 aneurysms in Budapest, with nine of the patients participating in the PITA study.15 Thirty-nine PEDs were implanted; one death (5.5%) occurred and there was one case of acute in-stent thrombosis with subsequent transient hemiparesis. McAuliffe and colleagues reported a prospective multicenter registry of 57 aneurysms in 54 patients treated with PED in Australia.16 They reported a total of 98 PEDs placed, with no major stroke or death. Fisher and colleagues reported a single-center case series of 88 patients with 101 aneurysms treated in Germany.20 One case of technical failure occurred and six patients (5.9%) developed major complications, including one death (1%). In our series, minor complications occurred in 12% of the cases, which is in line with the rate of 11% reported by Lylyk and colleagues.14 Furthermore, our 3% rate of major complications falls in the middle of the 0–5.9% range reported in these other series.
Technical aspects
Although the PED is labeled a ‘device’, its handling and deployment is truly a combination of multiple novel ‘techniques’ that require skilled maneuvers by the neurointerventionalist. As with most complicated procedures, there is certainly a learning curve for identifying cases that are technically suitable for PED embolization and for performing these cases. This learning curve is exemplified by two variables in our series: the mean fluoroscopy time and the number of cases requiring removal of a partially deployed PED. Both of these indirect markers showed improvement from the first half of the anterior circulation cases to the second half of cases. There was a 30% decrease in the mean fluoroscopy time when the first 15 anterior circulation cases (53.1 min) were compared with the second set (37.4 min) of the series. Similarly, all five cases that required removal of a partially deployed PED were in the first half of the series. Although case complexity will certainly affect both the fluoroscopy time and the success rate of PED deployment, the fact that there was improvement in both of these variables over the course of the series, which was performed by a single interventionalist, implied improved operator experience.
In our series a triaxial support system was used in 34/35 (97%) of cases, as this provides the necessary support and distal access for manipulation and deployment of the PED. However, a biaxial system was used successfully in a single case via a brachial approach for a posterior circulation aneurysm. The length of the Marksman catheter can also be a factor in safe and effective PED deployment. In our series, the 150 cm Marksman was used in 25 of 35 cases (71%). The longer Marksman catheter was often selected because it was typically used to track the guide catheter into its distal position rather than tracking the guide catheter over a standard glidewire (0.035 inch). The 150 cm Marksman catheter also facilitated opening the PED in the M1, which was commonly chosen because it is often a straight segment, and in recapturing the protective coil once the PED was deployed.
Complication avoidance
The single major complication in our series of acute SAH 5 h after PED implantation and subsequent death occurred following treatment of a wide-necked 3 mm aneurysm with a single PED. From a technical standpoint, the embolization was uneventful and the PED was implanted without any significant challenges. The rupture event raises questions regarding the precipitating factors that can lead to aneurysm rupture following PED placement.
Aneurysm rupture has been previously reported following PED treatment of both unruptured and previously ruptured aneurysms.15 ,20 ,25 ,26 For example, Fisher and colleagues reported a fatal aneurysm rupture of a 14 mm wide-necked paraclinoid aneurysm 3 days following embolization with three telescoped PEDs and adjuntive placement of a coil.20 Similarly, Hampton and colleagues reported SAH and death of a patient 5 days after embolization of a 35 mm ICA termination aneurysm with a single PED, despite having significant contrast stasis on immediate post-implantation control angiography.25 Szikora and colleagues reported a fatal aneurysm rupture 5 h following PED embolization of a giant proximal ICA aneurysm, but autopsy results suggested that the rupture was from a separate small aneurysm distal to the original target aneurysm. McAuliffe and Wenderoth reported two deaths from aneurysm rebleeding after PED embolization of recently ruptured aneurysms in a series of 11 patients.26 One patient had a 21 mm superior hypophyseal aneurysm treated with three PEDs and the other patient had a 34 mm dorsal paraclinoid aneurysms treated with two PEDs. Neither patient was treated with adjunctive coils. The rupture case in the current series, however, is the first reported case of small aneurysm (3 mm) rupture within hours after PED deployment.
Treatment of an aneurysm by flow diversion does not immediately exclude the aneurysm from the stresses of the arterial circulation, and there is a period when there is a risk of rupture until neoendothelialization of the PED occurs. The steps leading up to aneurysm rupture following flow diversion is probably a complex multifactorial process, with different factors involved in acute versus delayed rupture and in the rupture of small aneurysms versus large/giant aneurysms. Various hypotheses have been proposed for the etiology of aneurysm wall destabilization and rupture following implantation of a flow diverter. These include altered intraluminal and intra-aneurysmal hemodynamics and also the proteolytic, inflammatory and ischemic effects that the endosaccular thrombus has on the aneurysm wall.25 ,27–29 However, further studies are needed to understand these processes better.
Two additional techniques commonly employed by PED users (including in our series) to provide additional aneurysm protection are the placement of multiple overlapping PEDs and the adjunctive use of coils. Multiple overlapping or telescoped PEDs increase the mesh density over the aneurysm neck, thereby facilitating the flow diverting properties of the construct. Adjunctive coils promote endosaccular thrombosis and theoretically provide increased protection of the aneurysm when combined with flow diversion. However, there are potential downsides to both of these approaches. Placement of multiple PEDs increases the technical difficulty of the case, prolongs the procedure time, and the additional metal in the parent artery is likely to increase the risk of intraluminal thrombosis. Adjunctive coil placement increases procedural risk of aneurysm perforation because of the need to access the aneurysm with a catheter and wire. Neither of these techniques leads to complete immediate angiographic occlusion of the aneurysm in all cases, a finding thought to correspond to maximal aneurysm protection. In the large series by Fischer and colleagues, more than one PED was used in 67 of 101 procedures (66%), but none of the aneurysms had complete occlusion immediately after PED placement.20 Szikora and colleagues treated four patients in their series with adjunctive coils and, while three of them had complete angiographic occlusion of the aneurysm at the end of the procedure, one did not.15 In our series, immediate post-procedure angiography demonstrated pronounced contrast stasis in two patients treated with coils but only mild stasis in the third patient treated with coils. Furthermore, neither of these techniques fully protects against aneurysm rupture. Fisher and colleagues reported rupture of an aneurysm despite treatment with multiple PEDs and adjunctive coils.20
The definition and incidence of reported minor complications following PED embolization are quite variable in the literature. No minor complications were reported in the 31 patients participating in the PITA trial.17 Lylyk and colleagues, in their series of 53 patients (six of which were in the PITA trial) reported an 11% rate of minor complications, including groin hematomas, contrast-induced rash and exacerbation of cranial neuropathies. We reported four patients (11%) with minor complications (minor stroke, cranial nerve palsy, transient neurological deficit and groin infection) and, in general, these were similar to other published series. Following the single incident of groin infection in one patient who had an Angio-Seal closure device (case #7), we adopted the policy of giving a single dose of intravenous antibiotics (typically cefazolin or clindamycin) prior to femoral puncture.
In-stent thrombosis is an ongoing concern in patients following implantation of a PED. Szikora and colleagues reported one case of acute intra-procedure in-stent thrombosis attributed to patient non-compliance with antiplatelet medications.15 Fisher and colleagues reported thrombosis in two patients at 5 and 63 days after treatment, with the second case occurring 22 days after stopping clopidogrel.20 Two patients in our series had acute in-stent thrombus formation, one at the end of the procedure and the other 2 h after the procedure. Both cases of thrombosis were treated and fully resolved with an intra-arterial ReoPro bolus followed by a 24 h intravenous ReoPro infusion and systemic heparinization. The thrombogenicity of the PED is a real concern, and prophylaxis requires strict adherence to a dual antiplatelet regimen, full intra-procedure systemic heparinization and, in some cases, post-procedure systemic heparinization. Post-procedure systemic heparinization was used following cases of aggressive vessel manipulation or implantation of multiple devices.
Platelet inhibition assays such as VerifyNow P2Y12 (Accumetrics, San Diego, California, USA) were not routinely used in our series. Although there is growing interest in performing these tests prior to implantation of a vascular reconstruction device or PED, the interpretation of the results of these tests is certainly not standardized. The ideal level of platelet inhibition prior to PED implantation has yet to be established, and the appropriate response to perturbation of this level is unknown. As such, the FDA approved use of the PED in the USA based on results of the PUFS trial which did not require platelet inhibition studies. Centers such as ours that use standardized doses of aspirin and clopidogrel without checking levels will serve as important controls to those institutions that have incorporated these studies into their routine protocols.
Close patient monitoring following PED implantation is especially important for favorable patient outcomes. It is our practice that all patients undergo a DynaCT scan without contrast at the end of the procedure to assess PED morphology but, more importantly, to rule out intracranial hemorrhage. None of our patients had hemorrhage on their DynaCT scan. Once a hemorrhagic event is excluded, the intra-procedure systemic heparin is allowed to wear off (assuming post-procedure heparinization is not used). The patient is then transferred to the neurocritical care unit where neurological examinations are performed every hour for the first night. Any change in neurological condition following this procedure is taken seriously and rapidly assessed, as it could represent a thrombotic or a hemorrhagic event. A patient with an acute change in neurological examination undergoes a CT scan of the head without contrast to evaluate for hemorrhage, followed by emergency angiography for potential thrombolysis if the CT scan is negative.
Vascular access site infections are rare following percutaneous endovascular procedures.30 We report one case of cellulitis at the femoral puncture site which developed 2 weeks after the procedure. An arterial closure device (Angio-Seal) was used for hemostasis in this patient. Although this infection could have been an isolated event, we now administer a single dose of intravenous antibiotics (cefazolin or clindamycin) at the beginning of each procedure as prophylaxis. After implementing this protocol, we have not had another case of infection. Our rationale for pre-puncture antibiotics is that the complex access systems used for these procedures commonly involve shuttle sheaths (90 cm), and these sheaths have more contact with the skin when advanced into position than a standard short sheath (10 cm). This additional skin contact might result in an increased risk of infection. Even though anecdotal evidence suggests that prophylaxis with antibiotics is working to prevent infection at the vascular access site, further studies will be needed to validate this hypothesis.
Conclusions
Flow diversion remains an exciting new frontier in neurointerventional aneurysm treatment and its applications and limitations are still being defined. The use of flow diverters such as the PED for endovascular embolization of intracranial aneurysms is still largely in its infancy, particularly in the USA. We report the first single-institution, single-user series of aneurysm treatment with the PED in the USA since its FDA approval in April 2011. Our series demonstrates that the PED can be used in a safe manner to treat intracranial aneurysms, particularly when a rigorous and uniform technique is adopted. The immediate procedural outcomes using this technique in our series appear quite promising, although long-term results will need to be assessed.
References
Footnotes
-
Competing interests ALC is a proctor for the Pipeline embolization device (Covidien, Mansfield, Massachusetts, USA) and consultant for Covidien. The other authors have no conflict of interest. No author received financial support in conjunction with the generation of this submission.
-
Ethics approval This study was conducted with the approval of the Institutional Review Board of Johns Hopkins Hospital.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.