Article Text
Abstract
Background Digital subtraction angiography (DSA) is the gold standard imaging for detection of in-stent restenosis (ISR) but there is limited literature on optimal non-invasive surveillance imaging. In this study, the ability of CT angiography (CTA) and MR angiography (MRA) compared with DSA in recognizing ISR was assessed.
Methods A single center database of patients treated with stent implantation for ICAD was accessed. All patients who underwent follow-up imaging with DSA paired with either MRA or CTA within 30 days were included. Two angiography readers and two non-invasive imaging readers measured restenosis with a submillimeter digital caliper. ISR was categorized as: none/minimal, mild (<50%), moderate (≥50–70%) or severe (≥70%). Analysis was performed with weighted κ statistics.
Results 17 cases of individual stents that underwent surveillance imaging with paired DSA and CTA and five stents with paired DSA and MRA were identified. Of those undergoing DSA and CTA, inter-reader agreement produced κ=0.68 (95% CI 0.40 to 0.95) for DSA and κ=0.75 (95% CI 0.55 to 0.95) for CTA. Agreement across CTA and DSA was κ=0.36 (95% CI 0.26 to 0.52). Of those undergoing DSA and MRA, inter-reader agreement produced κ=0.71 (95% CI 0.27 to 1.00) for DSA and κ=1.00 (95% CI 1.00 to 1.00) for MRA. Agreement across MRA and DSA was κ=0.34 (95% CI 0.18 to 0.51).
Conclusions Good inter-reader agreement exists within DSA, CTA and MRA. However, when comparing non-invasive imaging (CTA and MRA) with DSA, only fair agreement exists. These data suggest that CTA and MRA are not comparable to DSA for evaluation of ISR.
Statistics from Altmetric.com
Introduction
Intracranial atherosclerotic disease (ICAD) is the cause of ischemic stroke in up to 10% of cases in the USA.1 It is even more common in Asia, accounting for 30–50% of strokes, making it the most common cause of stroke worldwide.2 Risk factors include Asian, African and Hispanic descent, insulin dependent diabetes mellitus, hypercholesterolemia, hypertension and smoking.3 The optimal therapy for patients is unknown and includes medical management, angioplasty and intracranial stenting. Patients with severe stenosis (70–99%) have a high risk of recurrent ischemic stroke despite maximum medical management, making this a subgroup that may benefit most from stenting.4 Rigorous appraisal of angioplasty with stenting for ICAD is limited, with one study showing no benefit with the use of the Wingspan stent system over medical management5 and another study ongoing.6 The strikingly high rate of recurrent ischemic stroke despite maximum medical therapy in patients with severe ICAD and the rapid pace of device design will likely contribute to continued investigation of angioplasty and stent implantation as a possible therapy.
For patients receiving stent placement, in-stent restenosis (ISR) has been documented in approximately 30% of cases.7 Digital subtraction angiography (DSA) is regarded as the gold standard for detection of stenosis8 and is currently the chosen modality for diagnosis of ISR.9 Due to the known complications of DSA,10 alternative imaging modalities for evaluating ISR have been explored.
Non-invasive modalities—namely, CT angiography (CTA) and MR angiography (MRA)—are limited by metallic and flow artifact, respectively.9 Researchers have studied various manipulations to protocol and processing of these modalities in a limited number of cases in order to maximize the sensitivity of detection for ISR.11–13 Clinical applications of these novel modifications remain experimental and are not readily available. Standard CTA and MRA have been documented for surveillance imaging after stent implantation7 ,8 ,14 in limited numbers of patients unable to undergo DSA. A comparison between standard non-invasive vascular imaging and DSA has not previously been reported.
We sought to compare the ability of CTA and MRA to detect the degree and location of ISR compared with DSA in patients receiving stent placement for ICAD.
Methods
Patient population
A database of patients treated with stent implantation for ICD from 2007 to 2010 was accessed following institutional review board approval. Per protocol, all patients underwent follow-up DSA imaging. Those who received a non-invasive imaging modality within 30 days of the DSA were considered to have a paired non-invasive and DSA imaging set and were included for analysis. Non-invasive modalities included CTA of the head, CTA of the head and neck, and MRA of the head.
Image acquisition
CT angiography
CTA of the head was obtained using a 64 channel multidetector GE LightSpeed CT scanner (GE Healthcare, Waukesha, Wisconsin, USA) with helical acquisition, slice thickness of 0.625 mm, pitch of 0.984:1 and table speed per rotation of 39.37 mm after intravenous injection of 65 ml of Omnipaque contrast material.
MR angiography
MRA of the head was obtained using 3 T Discovery, 1.5 T Optima, 1.5 T Signa Excite (GE Healthcare) as well as 3 T Verio and 1.5 T Espree (Siemens, Erlangen, Germany) MR scanners. Three-dimensional time of flight is the non-contrast angiographic method used across all scanners. The GE scanners acquire 34 locs per slab at 1.4 mm slice thickness with 50% overlap, while each of the Siemens scanners acquire 40 slices per slab at 0.6 mm slice thickness on the Verio and 0.7 mm on the Espree. The number of slabs and distance of overlap between adjacent slabs also vary across scanners: three slabs with 6.3 mm overlap on the Discovery and Optima, nine slabs with 6.3 mm overlap on the Excite, five slabs with 4.8 mm overlap on the Verio and four slabs with 5.6 mm overlap on the Espree. In terms of resolution, the field of view and matrix size vary in the following manner: 18 cm and 384×224 on the Discovery, 18 cm and 320×224 on the Optima, 16 cm and 220×220 on the Excite, 20 cm and 320×320 on the Verio and 18 cm and 256×241 on the Espree. In all cases, one NEX (average) is obtained, and the standard HNP coil is used.
Contrast enhanced MRA was also obtained after intravenous injection of 20 ml Multihance contrast material. The GE scanners acquire 48 locs per slab at 1 mm slice thickness with 50% overlap, while the Siemens scanners acquire 64 slices per slab at 1 mm slice thickness. Only one slab is acquired during this technique. In terms of resolution, the field of view and matrix size vary in the following manner: 20 cm and 320×224 on the Discovery and Optima, 20 cm and 256×224 on the Excite and 18 cm and 256×218 on the Verio and Espree. In all cases, one NEX (average) is obtained, and the standard HNP coil is used.
Digital subtraction angiography
Angiograms were performed using biplane DSA according to standard protocols at our institution. All patients received conscious sedation. A diagnostic 5 French catheter was used for manual contrast injections of non-ionic contrast material (iohexol; Omnipaque 300, GE Healthcare, Princeton, New Jersey, USA). Standard anteroposterior and lateral biplane projections were obtained for each angiogram and additional oblique views were obtained in the working projection from the time of stent implantation. Images were acquired at a frame rate of 2 frames/s.
Image analysis
Two DSA readers and two non-invasive (CTA and MRA) readers recorded the degree of ISR and location of ISR blinded to the original interpretations of the images. All measurements were made using a submillimeter digital caliper on a Picture Archiving and Communications System (PACS) workstation. Measurement technique varied slightly with imaging modality to reflect standard practice. In review of DSA, measurements were made using the optimal projection, as chosen by the investigator. Measurement of normal artery lumen caliber adjacent to the stent was used as the normal artery diameter, and the region of most severe narrowing within the stent was used for measurement of stenosis. In review of CTA and MRA, similar luminal assessment was performed and compared in axial, coronal and sagittal reformations with individual window level adjustments made by the reader to optimize imaging. These techniques were taken to reflect the normative real life approach. Degree of ISR was then categorized into one of four categories: 1=none/minimal, 2=mild (<50%), 3=moderate (50–69%) and 4=severe (≥70%). Readers submitted one measurement of each lesion for each of the modalities.
Statistical analysis
Cohen's kappa and weighted κ statistics were used to measure strength of agreement between readers, both within and between modalities. Overall κ values and tests for equal κ between strata were calculated using the method of Fleiss et al.15 Multiple scans of the same stent were weighted so that each stent contributed equally to the analysis. This weighted data should not be confused with weighted κ statistic. Also, some ratings were given as either of two categories (ie, ‘1 or 2’); these were split into two observations with a weight of one-half for each category. The analysis was performed using SAS V.9.2 (SAS Institute). ISR categories 1 and 2 were combined, as were categories 3 and 4, and a simple percentage of the time in which readers agreed within these combined categories is also reported. We also isolated category 4 (stenosis ≥70%) and reported percentages of agreement and disagreement.
Results
Over the period reviewed, there were 262 cases of stent implantation for ICAD. Of these, 40 cases underwent DSA and had either CTA or MRA performed within 30 days. This group consisted of 14 patients with 17 stents. Stents with repeated imaging had their results weighted and counted once. Overall, 17 stents underwent follow-up imaging with DSA and CTA within 30 days and five stents underwent DSA and MRA within 30 days. Patient demographics and stent characteristics are summarized in table 1. Representative images are shown in figures 1 and 2.
Patient demographics and stent characteristics
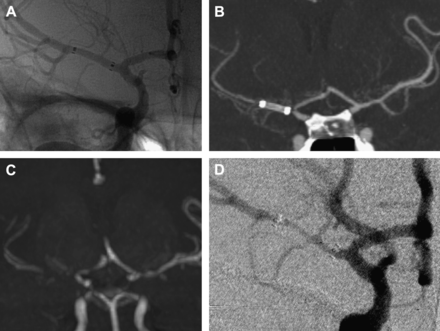
(A) Immediate post-stent implantation digital subtraction angiography (DSA) demonstrates a Wingspan stent within the right posterior cerebral artery. (B) Surveillance CT angiography (CTA) at 6 months shows the stent with evaluation of lumen caliber limited by stent artifact. (C) Follow-up DSA at 7 months demonstrates severe in-stent stenosis.
(A) Immediate post-stent implantation unsubtracted angiogram demonstrates a Wingspan stent in the right middle cerebral artery. (B) Surveillance imaging performed at 5 months shows stent artifact on CT angiography and (C) signal loss on MR angiography. (D) At the region of the stent. Follow-up digital subtraction angiography at 5 months characterizes narrowing within the stent.
Digital subtraction angiography versus CT angiography
Of those undergoing DSA and CTA (n=17), all included stents were Wingspan stents. Lesion locations included the internal carotid artery (ICA) (n=5), middle cerebral artery (n=6), posterior cerebral artery (n=3), anterior cerebral artery (n=1), intracranial vertebral artery (n=1) and basilar artery (n=1). For within DSA reads, inter-reader agreement produced κ=0.68 (95% CI 0.40 to 0.95) for all categories of ISR. For within CTA reads, inter-reader agreement produced κ=0.75 (95% CI 0.55 to 0.95) for the four categories of ISR. When comparing DSA with CTA, there was overall agreement of stenosis on 38.7% of stents with κ=0.39 (95% CI 0.26 to 0.52) for the four categories of ISR. Further analysis across DSA and CTA showed that readers agreed 36.9% of the time whether a stent belonged to ISR category 1 or 2, and 91.2% of the time within ISR category 3 or 4. Readers, therefore, agreed on 62.9% of stents as being characterized as either <50% stenosis (ISR category 1 or 2) or >50% (ISR category 3 or 4). We also studied the results within category 4 only, severe stenosis, and found that CTA was able to identify 85% of severely stenotic stents, as called by DSA. Conversely, 41.5% of stents were identified as severely stenotic by CTA when DSA did not categorize them as such. Tables 1 and 2 summarize the results and patient demographics of the DSA versus CTA subgroups.
Results of weighted κ statistics for degree of stenosis, digital subtraction angiography versus CT angiography
Digital subtraction angiography versus MR angiography
Of those undergoing DSA and MRA (n=5), all included stents were Wingspan stents. Lesion locations included the ICA (n=1), middle cerebral artery (n=2), posterior cerebral artery (n=1) and anterior cerebral artery (n=1). For within DSA reads, inter-reader agreement produced κ=0.71 (95% CI 0.27 to 1.0) for the four categories of ISR. For within MRA reads, inter-reader agreement produced κ=1.0 (95% CI 1.0 to 1.0) for the four categories of ISR. When comparing DSA with MRA, there was overall agreement of stenosis for 25.0% of stents with a κ=0.34 (95% CI 0.18 to 0.51) for the four categories of ISR. Further analysis across DSA and MRA showed that readers agreed 54.5% of the time whether a stent belonged to ISR categories 1 or 2, and 100% of the time for ISR categories 3 or 4. Readers, therefore, agreed on 75% of stents as being characterized as <50% stenosis (ISR category 1 or 2) or >50% (ISR category 3 or 4). We also studied the results within category 4 only, severe stenosis, and found that MRA was able to identify 100% of severely stenotic stents, as called by DSA. Conversely, 66.7% of stents were identified as severely stenotic by MRA when DSA did not categorize them as such. Tables 1 and 3 summarize the results and patient demographics of the DSA versus MRA subgroups.
Results of weighted κ statistics for degree of stenosis, digital subtraction angiography versus MR angiography
Discussion
This series presents a unique comparison of two non-invasive imaging modalities and DSA for surveillance imaging of intracranial stent implantation for ICAD. Similar efforts have been investigated for the use of CTA in coronary stents with varying degrees of success.16–19 Better visualization of coronary stent patency appears to be correlated with increased stent diameter and decreased stent strut size19 but the usefulness of CTA for evaluation of coronary, as well as intracranial, stents is still unclear.
Previous intracranial stent models were shown to overestimate the degree of ISR on CTA imaging.20 The composition of the newer Wingspan stent, however, allows for theoretically better visualization due to a low metal surface area and a thin strut size which decreases beam hardening and blooming effects, respectively.8
MRI has also played a role in the identification of coronary stent patency through the measurement of flow velocity.21 Comparable techniques have also been applied to intracranial stents in a small number of patients through the application of quantitative MRA with promising results.12 ,22 Although successful evaluation of ISR with standard MRA has been documented, the evidence is limited to two patients, one of which demonstrated complete occlusion while the other demonstrated adequate flow enhancement and was classified as ‘no ISR’.7
In our attempt to provide further evidence into the use of widely available non-invasive imaging techniques for stent imaging, we recruited four readers; two interpreted DSA images while the remaining two interpreted CTA and MRA images. We found good inter-reader agreement for evaluation of ISR within each respective modality (DSA, CTA and MRA). However, when comparing across modalities, CTA and MRA produced only fair agreement compared with DSA, with weighted κ coefficients of 0.39 and 0.34, respectively. This indicates that non-invasive imaging of intracranial stents may be reliable but its precision does not appear to be acceptable compared with the current gold standard, DSA. We were unable to calculate sensitivity and specificity due to multiple levels of outcomes, weighted observations and too small of a sample size for receiver operating curve analysis.
Subsequently, we sought to determine whether a trend for agreement existed with more lenient group pairing of degree of ISR. We combined ISR categories 1 and 2 to represent <50% restenosis, and also combined categories 3 and 4 to represent >50% stenosis. Using these parameters, readers agreed 62.9% of the time whether stenosis was <50% or >50% on DSA and CTA. The results were better within DSA and MRA in which readers agreed 75% of the time whether stenosis was <50% or >50%. While these results cannot be statistically tested, they may represent a trend that non-invasive imaging can only moderately differentiate <50% stenosis (categories 1 and 2) from >50% stenosis (categories 3 and 4). MRA, however, seems to have a stronger agreement than CTA compared with DSA.
We also selectively reviewed the severe stenosis (≥70%) category due to greater clinical relevance. We found that CTA was able to identify 85% of the stents that DSA determined to be severely stenotic. In contrast, 41.5% of stents were read as severely stenotic by CTA but were not categorized as such by DSA. Similar analysis for MRA showed that 100% of severely stenotic stents by DSA were correctly identified by MRA. However, MRA read 33% of the stents as severely stenotic when DSA did not. Although the patient sample was very small, these trends suggest that, collectively, non-invasive studies may tend to produce a significant false positive rate, with CTA being more prone to error than MRA. Sample size was limited by the study design. Patients were only included if they had a catheter angiogram with a non-invasive angiography study within 30 days for comparison. Although these inclusion criteria limited the sample size, this would not seem to introduce a selection bias that would confound the results. Of note, if our sample size permitted, it would have been interesting to analyze whether the variety of MR scanners with slight variation of technique had any influence on the results.
Limitations of our study include lack of valid advanced statistical analysis due to sample size. Head and neck CTA compared with head CTA may also have an appreciable difference in diagnostic yield due to differences in bolus time and image quality. Other important considerations for future studies include patient age, time since implantation, stent size and stent location. This study lacks the power to comment on the importance of these factors. We also allowed our readers to adjust window levels by their own judgment of optimum image contrast to simulate the normative real life approach. Results may have varied with standard windowing protocol.
ISR may be more common in patients aged 55 years and younger and those with stents in the supraclinoid ICA.23 As our understanding for risk factors of ISR evolves, more specific selection of follow-up imaging for ISR may be possible. Advancements in imaging may also provide better insight into the imaging modality of choice. Optimized sharp kernel CT,24 rotational angiographic CT25 and quantitative MRA12 ,22 have all been studied in evaluation of ISR with limited but promising results.
Conclusion
Our results suggest that CTA and MRA for surveillance of ISR compare poorly with DSA, the current gold standard. Although non-significant, MRA seems to have a stronger agreement than CTA compared with DSA, while both tended to have a higher rate of false positives when evaluating severely stenotic stents. These data suggest that in the current state of imaging acquisition techniques and stent technology and composition, non-invasive imaging is limited in ability to evaluate ISR. Better understanding of ISR and improvement of current imaging modalities may allow for patient specific guidelines for imaging modality.
References
Footnotes
-
Competing interests None.
-
Ethics approval Ethics approval was provided by the institutional review board of the Medical College of Wisconsin.
-
Provenance and peer review Not commissioned; externally peer reviewed.