Article Text
Abstract
Background Endovascular treatment of intracranial aneurysms via flow diversion has become increasingly popular over the past several years. The flow redirection endoluminal device (FRED; Microvention, Tustin, California, USA) system is a next generation closed cell paired stent flow diversion device.
Objective Our initial clinical experience with the FRED system is described. We believe this series to be the first use of the FRED system in the western hemisphere.
Methods 14 aneurysms were treated utilizing the FRED system in 13 patients. Post-deployment angiography and fluoro CTs were obtained in all cases.
Results Immediate post-treatment angiography demonstrated reduced flow into all aneurysms although no long term angiographic data are yet available. The device proved technically easy to deploy and recapture after partial deployment if needed. No complications, technical or otherwise, were encountered. Radiographic visibility and ability to maintain its internal cylindrical shape in tortuous arteries, as demonstrated by fluoro CT, was at least as good as the pipeline embolization device.
Conclusions The FRED system was technically easy to deploy with no procedural complications occurring in this first reported series of 14 aneurysms. The ability of the FRED system to be recaptured after partial deployment and to maintain its internal shape in tortuous vessels was demonstrated well. Long term clinical and angiographic follow-up along with prospective studies are now needed to ascertain the role of the FRED in intracranial aneurysm treatment.
- Aneurysm
- Angiography
- Device
- Flow Diverter
- Intervention
Statistics from Altmetric.com
Introduction
Endovascular treatment of intracranial aneurysms via flow diversion is based on changing the hemodynamic factors associated with aneurysm development and growth by placing a finely meshed stent-like device across the neck of the aneurysm. Endoluminal vessel reconstruction occurs as the device undergoes endothelialization via neointimal growth, as opposed to only endosaccular exclusion achieved with coiling.1 ,2 The concept was postulated almost 20 years ago3 but has only become widely utilized recently with the introduction of the silk flow diverter (SFD) and pipeline embolization device (PED). Prior to these constructs, endovascular neurointerventionalists were occasionally using coronary stents and intracranial stents as definitive monotherapy treatment for otherwise untreatable aneurysms.4–6 However, the meshing in these stents was much more porous compared with the first true flow diverters. The PED and SFD provide coverage of 30–35% and 35–55% of the inner vessel surface, respectively.7 The PED has been shown to achieve total angiographic occlusion in 52–100% of aneurysms treated, with periprocedural mortality and morbidity of 0–5% and 6.5–13.9%, respectively, in most series.2 ,8–17 The mortality rate increases significantly (∼18%) when ruptured aneurysms are specifically evaluated.13 The early experience with the SFD has shown mortality rates of 4–8%, permanent morbidity of 4–15%, with total angiographic occlusion achieved in 49–69% of cases.18 ,19
The flow redirection endoluminal device system (FRED; MicroVention, Tustin, California, USA) is a next generation flow diversion device designed for the treatment of intracranial aneurysms. The construct consists of a compliant closed cell paired stent (aka ‘stent within a stent’) composed of single wire braid self-expanding nickel titanium. Its fluoroscopic visibility is marketed as being superior to first generation flow diverters by virtue of radiopaque markers at the proximal and distal ends as well as interweaved radiopaque helical marker strands along the entire working length of the device. Resheathing after up to 50% partial deployment is also possible. We report here our initial experience with the FRED, which we believe represents its first use in the western hemisphere.
Materials and methods
Fourteen aneurysms were treated in 13 patients during the months of February and May 2013. Demographics and aneurysm details are shown in table 1. Patients were predominately female (10/13, 77%), and mean age was 57 years. Eight of the 13 patients (62%) had suffered a previous aneurysmal hemorrhage (seven subarachnoid hemorrhage, one brainstem hemorrhage) from either the aneurysm treated or another aneurysm. Only one aneurysm was located in the posterior circulation. The majority of aneurysms were small in size (8/14, 57%). Six of the 14 aneurysms (43%) had undergone previous treatment prior to treatment with the FRED system. All cases were performed by a single endovascular neurointerventionalist in February and May 2013.
Patient demographics, aneurysm characteristics, and FRED device utilized to treat the aneurysm(s) in question
All patients underwent transfemoral cerebral angiography under general anesthesia with three-dimensional reconstructions obtained to allow for accurate measurements of the internal carotid artery. Aspirin and clopidogrel were given 7 days prior to each procedure although no accumetrics were available. The internal carotid artery diameter proximal to, at, and distal to the aneurysm was measured as well as the entire length of artery the FRED was to be deployed in. The ideal FRED diameter is the maximum diameter of the artery it is being deployed into, usually the arterial diameter proximal to the aneurysm. A Chaperon guide catheter (Microvention), Headway 27 microcatheter (Microvention), and Traxcess 14 microwire (Microvention) were used in all cases. The deployment technique involves advancing the Headway microcatheter distal to the aneurysm and partially deploying the initial part of the FRED. The construct is then pulled back to the desired position. The FRED is then slowly deployed under constant fluoroscopy ensuring that there is good apposition of the stent against the vessel lumen. Two separate angiographic views are used to assure this. Satisfactory placement is confirmed with fluoro CT. The aneurysm should be centered within the FRED construct, and only the working length (ie, the overlapping outer/inner stent length) of the FRED should cover the aneurysm to ensure flow diversion. The working length of the FRED includes only the overlapping outer/inner stent length, and thus there is 2–4 mm at each end of the device that represents only the outer stent length.
Results
Fluoro CT images obtained at the conclusion of each case showed excellent covering across all aneurysm necks, with preservation of the internal cylindrical shape of the device (ie, no device kinking). Immediate flow reduction was noted in all cases on immediate post-deployment angiography. There were no technical problems encountered with the device and there were no immediate complications suffered by the patients. Representative cases are shown and described in figures 1⇓–3.
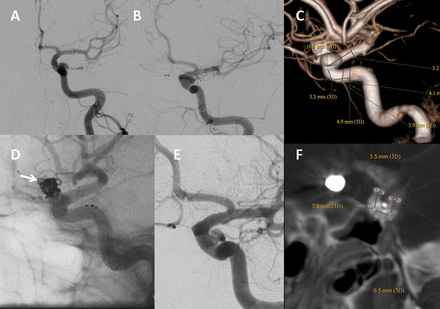
Patient No 2. Left ophthalmic segment aneurysm is shown in anteroposterior (A), oblique (B), and lateral three-dimensional (C) projections. Measurements shown in (C) were done for the purposes of choosing a correct device size. (D) Unsubtracted left internal carotid artery injection immediately after flow redirection endoluminal device (FRED) deployment, demonstrating satisfactory placement. The white arrow indicates the coils present in the contralateral ophthalmic aneurysm. (E) An immediate post-treatment oblique projection. The flow in the aneurysm has decreased compared with (B). The bottom right of (F) shows a fluoro CT image demonstrating maintenance of the internal cylindrical shape of the device around the tortuous carotid siphon.
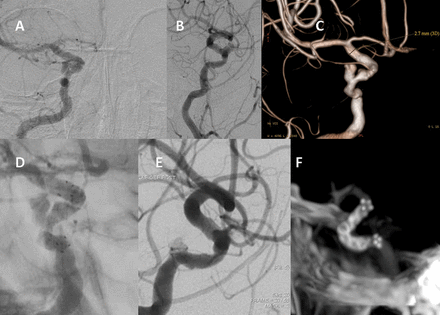
Patient No 3. Right paraophthalmic segment aneurysm is shown in anteroposterior (A), lateral (B), and oblique three-dimensional (C) projections. Measurements shown in (C) were done for the purposes of choosing the correct device size. (D) Unsubtracted right internal carotid artery injection, oblique projection, immediately after flow redirection endoluminal device (FRED) deployment, demonstrating satisfactory placement. (E) Immediate post-treatment lateral projection. The flow in the aneurysm has decreased compared with (B). The bottom right of (F) shows a fluoro CT image, demonstrating maintenance of the internal cylindrical shape of the device around the tortuous carotid siphon.
Patient No 8. Right paraophthalmic and superior hypophyseal artery aneurysms are shown in anteroposterior (A), lateral (B), and lateral three-dimensional (C) projections. Immediate post-flow redirection endoluminal device (FRED) deployment unsubtracted lateral (D) view is shown, demonstrating the FRED covering both aneurysm necks. Post-treatment lateral projection (E) demonstrating reduction in aneurysmal blood flow. (F) Fluoro CT image demonstrating maintenance of the internal cylindrical shape of the device around the tortuous carotid siphon.
Discussion
We found the FRED to be relatively easy to deploy compared with the PED in this initial experience. Radiographic visibility was as good as the PED. The ability of the device to maintain its internal diameter and cylindrical shape without bending and kinking around the tortuous carotid siphon also appears to be a technological advancement of this device compared with the older generations of stents and flow diverters. We saw an immediate change in aneurysmal blood flow after FRED deployment although we currently have no follow-up to report on aneurysm thrombosis rates. No technical or clinical complications were experienced in this small series, which is encouraging considering the high rate of deployment difficulties initially reported in some series with other flow diverters, such as the SFD.18 We will be interested to see to what extent the complications associated with the earlier flow diverters (intraparenchymal hemorrhage, stent thrombosis, thromboembolic phenomena, parent artery stenosis, delayed aneurysmal rupture, delayed in-stent stenosis, etc) will be encountered with the FRED.7 ,20 Weaknesses of this paper include the small number of cases treated, the lack of long term angiographic follow-up, and the predominance of small aneurysms treated.
Conclusion
Endovascular vessel reconstruction via flow diverting devices is an increasingly popular approach to treating intracranial aneurysms. The FRED system was technically easy to deploy with no procedural complications occurring in this first reported series of 14 aneurysms. The ability of the FRED to be recaptured after partial deployment and to maintain its internal shape in tortuous vessels was demonstrated well. Larger prospective series with long term clinical and angiographic follow-up will be needed to fully ascertain the role this device will play in the treatment of intracranial aneuryms.
References
Footnotes
-
Contributors OD performed all procedures and contributed to manuscript conception, design, creation, editing, and revision. TLG contributed to manuscript conception, design, creation, editing, and revision. GM, FO, and RA contributed to manuscript conception and design.
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval The study was approved by the institutional review board of Madre Bernarda Hospital, Cartagena, Colombia.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement Additional unpublished angiographic and fluoro CT data for each case are available to readers of the journal. Please email all requests to odiaz@houstonmethodist.org