Article Text
Abstract
Background In order to determine the risk factors related to aneurysm rupture, we studied the aneurysms at the paraclinoid segment of the internal carotid artery by applying morphologic and hemodynamic numerical analyzes.
Methods 107 patients with 110 paraclinoid aneurysms (26 ruptured, 84 unruptured) were analyzed using computational fluid dynamics based on patient-specific three-dimensional geometrical models. A series of morphologic and hemodynamic parameters were evaluated to find the potential indicators of aneurysm rupture.
Results Aneurysms with an irregular shape accounted for 23.1% of the ruptured group and only 8.3% of the unruptured group. The difference was statistically significant (p=0.042, χ2 test). Ruptured paraclinoid aneurysms were found to be significantly smaller than unruptured aneurysms (p=0.041), which is different from the results of most previous studies. Energy loss (EL) and inflow concentration showed a level of statistical significance to assess the risk of rupture in paraclinoid aneurysms. By multivariate logistic regression analysis, aneurysm shape (regular or irregular), EL and inflow concentration were retained as independently significant parameters. The odds of rupture were increased by 1.65 times for a 10% increase in EL, by 4.88 times for an aneurysm with an irregular shape and by 2.91 times for an aneurysm with concentrated inflow jet.
Conclusions Irregular shape, larger EL and concentrated inflow jet were independently associated with the rupture status of paraclinoid aneurysms. These findings need to be further confirmed based on large multicenter and multipopulation data.
Statistics from Altmetric.com
Introduction
Paraclinoid aneurysms are defined as aneurysms arising from the segment of the internal carotid artery (ICA) between the distal dural ring and the origin of the posterior communicating artery.1 ,2 It has been reported that paraclinoid aneurysms account for approximately 5–14% of all intracranial aneurysms.3 ,4 With the technological advancements in vascular imaging, more asymptomatic paraclinoid aneurysms are found incidentally. Several studies5–7 and the experience of our team suggest that more than two-thirds of paraclinoid aneurysms are unruptured, with or without clinical symptoms.8 The final decision on asymptomatic unruptured paraclinoid aneurysms is a trade-off between the perceived risk of rupture and the risk of treatment complications. A comparison of morphologic and hemodynamic factors between ruptured and unruptured paraclinoid aneurysms is therefore of great value, and the findings may provide an important reference for physicians.
Computational fluid dynamics (CFD) analysis has been widely used in recent studies of the risk of intracranial aneurysm rupture by probing several qualitative and quantitative parameters that are recognized as crucial factors responsible for aneurysm rupture.9–15 Unfortunately, paraclinoid aneurysms have not been included or only accounted for a small proportion of the samples. To our knowledge, only a few researchers have specifically examined the hemodynamic characteristics of paraclinoid aneurysms based on three-dimensional (3D) patient-specific models. However, their sample sizes were relatively small and the findings were conflicting.10 ,16 ,17 Many researchers are still plagued by the ‘high or low wall shear stress (WSS)’ controversy.18
As with hemodynamic variables, correlations between aneurysm morphologic parameters and the risk of rupture have also been reported14 ,19–21 but, like hemodynamic studies, the morphologic results have not always been consistent. Most large sample studies included different locations of aneurysms, which may be one of the reasons for the conflicting results. Given the controversial findings from these studies, we believe it is necessary to clarify the hemodynamic conditions and morphologic features associated with rupture of aneurysms specifically located in the paraclinoid segment of the ICA using large sample sizes.
In the present study we combined the morphologic measurements with patient-specific 3D models to describe the qualitative and quantitative hemodynamic characteristics in 110 paraclinoid aneurysms. To our knowledge, the sample size of our study is the largest to date. Our aim was to explore the potential mechanisms associated with aneurysm rupture on the basis of the characteristics of paraclinoid aneurysms. Surprisingly, an unusual finding was obtained from the comparison of ruptured and unruptured paraclinoid aneurysms.
Materials and methods
Patients
From November 2008 to July 2012, 107 patients with 110 paraclinoid aneurysms from our database were retrospectively examined and included in our study. All of them had 3D digital subtraction angiography (DSA) images of adequate quality and complete clinical data. The 110 aneurysms were divided into two groups: ruptured (26 aneurysms) and unruptured (84 aneurysms). If more than one aneurysm was found in the presence of hemorrhage, the distribution of subarachnoid blood was used to identify the aneurysm that had ruptured. If a clear decision could not be reached, the patient was excluded. The population consisted of 87 women and 20 men of mean age 52.1 years (range 23–74).
Modeling of the aneurysms and numeric simulation
Patient-specific 3D-DSA data of all paraclinoid aneurysms were obtained before treatment. 3D surface reconstruction was completed using standard proprietary software into a standard tessellation language format. Before meshing, the aneurysm and a 20 mm long segment of surrounding parent artery were trimmed out for CFD analysis and defined as ‘aneurysm (AN)’ and ‘parent artery (PA)’, respectively. Each model was imported into the ICEM CFD software (ANSYS, Canonsburg, Pennsylvania, USA) to create more than one million finite volume tetrahedral element grids. After meshing, ANSYS CFX V.11.0 software was used for simulation of blood hemodynamics as previously described.22 Two cardiac cycle simulations were performed for numeric stability. The results from the second cardiac cycle were collected as output for the final analyzes.
Calculation of morphologic and hemodynamic parameters
Four morphologic parameters (size, area of AN, aspect ratio (AR), size ratio (SR)) as described previously by Dhar et al20 were measured from 3D angiography image data. Aneurysm shape was classified either as regular or irregular. Irregular aneurysms were defined as aneurysms with multiple irregularities due to bleb formations. After CFD simulation, the following quantitative hemodynamic parameters were calculated at peak systole: spatial average and maximum WSS at AN (aWSS, mWSS), spatial average WSS at PA, low WSS area (LSA), ratio of low WSS area and energy loss (EL). Oscillatory shear index (OSI) values were computed over the second cardiac cycle.
In this study, LSA was defined as the area of the aneurysm wall exposed to a WSS below the threshold which was 10% of the mean parent arterial WSS.10 ,14 EL represents the expenditure of flow energy in the aneurysm region and was considered to be associated with aneurysm rupture.12 ,13 EL was expressed as: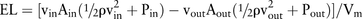 where Vm represents the volume of the measurement section, Ain, vin and Pin represent the area, mean flow velocity and static pressure of the test plane at the inlet side and Aout, vout and Pout represent those at the outlet. ρ is the fluid density.13
where Vm represents the volume of the measurement section, Ain, vin and Pin represent the area, mean flow velocity and static pressure of the test plane at the inlet side and Aout, vout and Pout represent those at the outlet. ρ is the fluid density.13
To allow for comparison between aneurysms with different sizes, EL was normalized by its volume of the measurement section13 (figure 1) and WSS was also normalized by the aWSS of their parent arteries.14
Extraction of the measurement section in patient-specific aneurysm model: the section between the inlet side (red) and the outlet side (gray) was the measurement section.
In order to understand the potential role of hemodynamics in aneurysm rupture, we also studied four qualitative hemodynamic characteristics that describe aneurysmal flow complexity and stability, inflow concentration and impingement zone using the method of Cebral et al.9
Data analysis
All quantitative morphologic and hemodynamic parameters were presented as means and SDs and were tested by the Kolmogorov–Smirnov test for normal distribution. A t test was used if a parameter was normally distributed, otherwise a Mann–Whitney test was performed to compare the differences between the two groups. For qualitative parameters, a χ2 test was used for statistical analysis. p<0.05 was regarded as statistically significant. The parameters found to be significant were further analyzed using multivariate logistic regression to help understand possible interrelationships between them.14 ,18 In order to facilitate odds ratio comparison, it was desirable that a unit increase in the parameter corresponds to 10% of its observed range. Therefore, each of the quantitative parameters was scaled to span a range from 0 to 10 before performing the regression. Statistical analysis was performed with SPSS V.17.0 (SPSS, Chicago, Illinois, USA).
Results
Common risk factors
In our study, no significant differences in common risk factors (age, gender, cigarette smoking, history of hypertension, multiple aneurysms) were found from χ2 tests between the ruptured and unruptured groups (table 1).
Comparison of common risk factors for aneurysm rupture between the ruptured and unruptured groups
Differences between ruptured and unruptured groups in morphologic measurements
None of the morphologic parameters were normally distributed according to the Kolmogorov–Smirnov test (table 2). AR was not significantly different between the two groups (p=0.379). The results of the Mann–Whitney test showed that aneurysm size, area of AN and SR were significantly different (p=0.041, p=0.048 and p=0.031, respectively); the size and area of ruptured aneurysms in our sample were significantly smaller than those of unruptured aneurysms (4.4131 vs 5.6287 mm and 83.1 vs 135.3 mm2, respectively, figure 2A).
Univariate analysis results for morphologic parameters in ruptured and unruptured groups
Comparison of aneurysm (AN) size and energy loss (EL) between ruptured and unruptured groups. (A) Differences in aneurysm size and area between ruptured and unruptured groups. The ruptured cases were significantly smaller than unruptured cases. (B) Comparison of EL from ruptured and unruptured aneurysms. The result showed that EL at peak systole in the ruptured group was two times (p=0.011) higher than in the unruptured group.
We also examined the difference in aneurysm shape which was regarded as an inherently qualitative feature between the two groups. The result showed that aneurysms with an irregular shape accounted for 23.1% of the ruptured group and only 8.3% of the unruptured group. This difference was statistically significant by the χ2 test (table 1).
Differences between ruptured and unruptured groups in hemodynamic measurements
From the Kolmogorov–Smirnov test, EL, aWSS, mWSS and aWSS at PA were normally distributed. The remaining parameters were not normally distributed. Their mean (SD) values are shown in table 3. The value of EL in the ruptured group was twice that in the unruptured group (71 441.23 W/m3 and 32 326.44 W/m3, respectively, figure 2B). Statistical analysis revealed an association between aneurysm rupture and EL (p=0.011). The remaining quantitative parameters were not significantly different between the two groups.
Univariate analysis results for hemodynamic parameters in ruptured and unruptured groups
In the ruptured group, aneurysms were more likely to have complex flow patterns (65.4%) and large impingement zones (61.5%), but the flow stability and concentration did not show significant differences. On the other hand, unruptured aneurysms were more likely to have stable flow patterns (70.2%), diffuse inflows (64.3%) and large impingement zones (72.6%), while the flow complexity was not significantly different. After χ2 test, only inflow concentration was associated with the risk of aneurysm rupture (p=0.046, table 4, figure 3).
Statistical analysis of the associations between qualitative hemodynamic parameters and aneurysm rupture
Wall shear stress distribution (left), streamline (middle) and velocity magnitude on a cut plane (right) at peak systole. The top row shows a ruptured case and the bottom row shows an unruptured case. A concentrated and high-speed inflow jet was observed in the ruptured case. There was a small bleb aligned to the inflow jet and the inflow impingement into the bleb. The flow pattern was complex in the aneurysmal sac. In the unruptured case, the inflow jet was diffuse and the flow pattern was relatively simple.
Multivariate logistic regression of significant variables
To assess the independent effect of variables on predicting rupture of paraclinoid aneurysms, multivariate logistic regression analysis was performed on those factors which were significant in univariate analyzes (aneurysm shape, aneurysm size, area of aneurysm, SR, EL and inflow concentration). The final parsimonious model for assessing aneurysm rupture risk (odds) was obtained: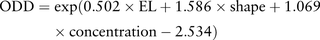 Aneurysm shape, EL and flow concentration were retained as independently significant factors. The odds of rupture increased by 1.65 times for a 10% increase in EL, by 4.88 times for an aneurysm with an irregular shape and by 2.91 times for an aneurysm with concentrated inflow jet.
Aneurysm shape, EL and flow concentration were retained as independently significant factors. The odds of rupture increased by 1.65 times for a 10% increase in EL, by 4.88 times for an aneurysm with an irregular shape and by 2.91 times for an aneurysm with concentrated inflow jet.
Discussion
The mechanisms of intracranial aneurysm rupture have been explored based on morphologic and hemodynamic characteristics,9 ,14 ,20 ,21 ,23 but aneurysms from different locations rather than from a single anatomic location were involved in these studies. This might be one of the reasons why some conflicting findings were reported. In an attempt to eliminate the potential biases from different locations, we focused on aneurysms at a single paraclinoid segment in the present study. We have found that paraclinoid aneurysms without subarachnoid hemorrhage (SAH) accounted for a large proportion, and the management of unruptured paraclinoid aneurysms is a complex problem due to the high risk of clinical intervention.8 We attempted to explore the potential clinical utility of morphologic and hemodynamic characteristics in guiding neurointerventional and neurosurgical treatment of paraclinoid aneurysms.
In terms of morphology, aneurysm blebs and irregular shapes were considered to be correlated with increased rupture risk.19 ,21 ,22 Our finding confirmed the notion that the irregular shape of aneurysms plays a positive role as an independent factor during the process of aneurysm rupture. Among the morphologic parameters, aneurysm size was most widely studied. Conventional studies deemed that larger aneurysms carry a higher risk of rupture.24 ,25 However, many small aneurysms have caused SAH and some larger ones maintained a stable situation. According to our present study, the notion that larger aneurysms have a higher rupture risk may not be applicable to paraclinoid aneurysms. Similar to the suggestion by Raghavan et al21 that a small irregular aneurysm might have a greater risk of rupture than a larger smoothly contoured aneurysm, we have found that smaller paraclinoid aneurysms are associated with rupture and the size difference between the two groups achieved a level of statistical significance. SR, defined as (maximum aneurysm height)/(average vessel diameter), takes into account aneurysm size and also the caliber of its parent vessel. It therefore indirectly accounts for the effect of parent vessel diameter via the SR.14 ,20 By univariate analyzes, SR and the area of aneurysm achieved a level of statistical significance, but they were not retained as significant in multivariate logistic regression analyzes. Meng et al18 demonstrated that SR was significantly larger in the ruptured group, which was different from our results (table 2). This remains to be studied further in larger cohorts from multiple centers.
While a cohort of 110 aneurysms is a small sample and biases are inevitable, our findings demonstrate possible characteristics that are specific to aneurysms at the paraclinoid segment. This might help us to understand the mechanisms of aneurysm rupture and assess the rupture risk of unruptured aneurysms.
Currently, the role of hemodynamics in aneurysm rupture is undergoing increasing amounts of research, and a growing number of hemodynamic parameters have been proposed as potential indicators of aneurysm rupture.15 WSS is a frictional force generated by the blood flow which tangentially impacts on the arterial lumen. It is believed to play an important role in the natural process of cerebral aneurysms. WSS is the most highlighted parameter in recent years, but the results have been controversial.11 ,14 ,26 ,27 Cebral et al26 ,27 found from their CFD simulations that ruptured aneurysms were more likely to have a larger WSS whereas a low WSS was thought to contribute to aneurysm rupture in other CFD studies.11 ,14 Because of the conflicting results as well as the large number of hemodynamic parameters in recent publications, some questioned the potential utility of CFD, a concern expressed in an editorial by Kallmes.28 On the other hand, Cebral and Meng29 argued that, in this exploratory phase of understanding aneurysm rupture, some initial divergence is unavoidable but convergence is necessary in order for CFD to realize its huge potential in clinical utility. Closing the gap requires both larger coordinated studies and increased understanding of the hemodynamic-biologic mechanisms. Based on extensive literature and experimental evidence, Meng et al18 proposed a new concept that both low and high WSS could drive intracranial aneurysm growth and rupture, albeit via different biologic mechanisms. The complexity and heterogeneity of aneurysm lesions might indicate different phenotypes, and therefore different pathways. Studying location-specific aneurysms such as paraclinoid aneurysms rather than aggregations of aneurysms from all locations may help to delineate such differences.
We have examined the association of rupture status of paraclinoid aneurysms with hemodynamics using quantitative hemodynamic parameters and also qualitative flow characteristics. We found that only EL and flow concentration remained as independently significant parameters after multivariate logistic regression analysis. EL was introduced as a parameter to describe the loss of flow kinetic energy within the aneurysm, and it was used to predict aneurysm rupture.12 ,13 Converting into heat, physical forces and pressure on pathologic aneurysm surface, EL is thought to be absorbed by the aneurysm wall resulting in endothelial cell degeneration, which could eventually lead to future rupture.12 ,13 Cebral et al9 ,23 performed qualitative hemodynamic analysis of aneurysms from different locations and found that ruptured aneurysms were more likely to have a complex and unstable flow pattern, concentrated inflow and small impingement zones. These qualitative parameters, including flow complexity, flow stability, inflow concentration and impingement zone, were used to describe intra-aneurysmal blood flow such as inflow streams, flow structures, flow impingement and changes in blood flow pattern.9 ,23 They suggested that the inflow jet and interaction with the aneurysm wall might play an important role in the process of aneurysm rupture. A concentrated inflow jet impacted the aneurysmal wall, producing a region of locally elevated WSS. This hemodynamic condition could lead to endothelial cell responses that elicit wall destructive remodeling, as demonstrated in experimental models,18 ,30 making the wall prone to rupture. For paraclinoid aneurysms, our results seem to support this notion. However, 61.5% of our ruptured aneurysms had large impingement zones, which was different from previous studies. Regardless of the results of our analysis, such issues are still unsettled until they are tested in larger studies.
The mechanisms of aneurysm rupture cannot be explained simply by hemodynamics or morphology. Genetic factors, clinical history and changes in pathophysiology are also considered to play an important role in the natural history of aneurysms. It is necessary to assess risk factors comprehensively in the future. Like other CFD simulation studies, rigid wall, laminar flow and Newtonian blood were used in our aneurysm models which could affect the reality of the hemodynamic result. Meanwhile, selection bias may be inevitable in data from only one medical center and our result may not be applicable to aneurysms from other centers. The findings of our research remain to be further verified in larger cohorts from multiple centers.
Conclusion
Irregular shape, larger EL and concentrated inflow jet were independently associated with the rupture status of paraclinoid aneurysms. These findings in paraclinoid aneurysms need to be further confirmed based on large multicenter and multipopulation data.
Acknowledgments
The authors gratefully acknowledge help in the analysis and interpretation of the data by Ding Ma, The Mechanical and Aerospace Engineering Department and Toshiba Stroke Research Center, University at Buffalo, State University of New York, USA.
References
Footnotes
-
Contributors JL and YW: drafting and revising the article. HM and HL: substantial contributions to the conception and design. JX and YZ: drafting the article, analysis and interpretation of the data. XY: conception and design, final approval of the version to be published.
-
Funding This study was supported by the National Science Foundation of China (Grant No. 81220108007 and 81171079), High-Level Health Technique Talent Training Plan of Beijing Health System (Grant No. 2009-3-22), National ‘Twelfth Five-Year’ Plan for Science and Technology Support (Grant No 2011BAI08B06) and National 973 Basic Research Program of China (Grant No. 2010CB732605).
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval This study was approved by the ethics committee of Beijing Neurosurgical Institute.
-
Provenance and peer review Not commissioned; externally peer reviewed.





