Article Text
Abstract
Background Flow diversion is an established technique for treatment of cerebral aneurysms. The Pipeline embolization device (PED) is the only FDA-approved flow diverting stent in the USA. A second-generation device, PED Flex, has recently been released with modifications to the delivery system. Published reports of experience with this new device are limited.
Objective To describe the initial outcomes from the first North American series using the PED Flex—a single-center experience of 44 cases.
Methods All patients consecutively treated with the PED Flex embolization device from February 2015 through April 2015 were included in the study. Data were collected for patient demographics, aneurysm characteristics, technical procedural details, and early outcomes.
Results PED Flex treatment was attempted in 42 patients (mean 56.6±2.0 years) with 44 aneurysms (mean size 6.5±0.6 mm), 41/44 (93%) of which were anterior circulation and 3/44 (7%) were posterior circulation. PED Flex was successfully implanted in 43/44 cases (98%). A single device was used in 41/43 cases (95%), with a mean of 1.07±0.05 devices implanted per case. Resheathing was performed in 4/44 cases (9%). Mean postprocedure hospital length of stay was 1.3±0.2 days. One significant neurological complication (2.3%) occurred, which was a stroke in a patient non-compliant with the prescribed antiplatelet regimen.
Conclusions Pipeline Flex is a second-generation flow diverter with enhanced features compared with the first-generation PED. These modifications allow for more reliable deployment with continued improvements in procedural safety.
- Aneurysm
- Device
- Flow Diverter
- Intervention
- Stent
Statistics from Altmetric.com
Introduction
Flow diversion with the Pipeline embolization device (PED; Covidien, Mansfield, Massachusetts, USA) is a well-established technique for treatment of intracranial cerebral aneurysms, particularly large and giant internal carotid artery (ICA) aneurysms. Its safety and efficacy has been demonstrated in numerous clinical studies, most notably the Pipeline for Uncoilable and Failed Aneurysms (PUFS) trial,1 the International Retrospective Study of Pipeline Embolization Device (IntrePED),2 and several large clinical series.3 ,4 Flow diversion with the PED also offers reduced radiation exposure5 and implant cost savings6 compared with traditional coiling techniques. Despite the evidence supporting excellent outcomes, these procedures are often challenged by difficulty in deliverability and opening of the cobalt–chromium PED implant.
The second-generation PED, named Pipeline Flex (PED Flex), received the European CE mark of approval in March 2014 and subsequently received Food and Drug Administration (FDA) approval on 5 February 2015. Its design includes numerous delivery system changes to enhance device opening and provide additional safety with a resheathing feature. To date, only three small clinical series from Europe7–9 and a single case report from North America10 have described its use. In this report, we describe the initial outcomes from the first North American series using the PED Flex, a single-center experience of 44 cases.
Patients and methods
Patients
All patients consecutively treated with the PED Flex embolization device at Johns Hopkins from February 2015 through April 2015 were included in the study. The procedures were performed by two neurosurgeons (ALC, GPC). Data were collected for patient demographics, aneurysm characteristics, technical procedural details, and immediate procedural outcomes.
Endovascular procedure and access system
Procedures were performed on a biplanar flat panel angiographic system (Artis zee, Seimens, Erlangen, Germany) under general anesthesia. Patients were treated preoperatively with aspirin 325 mg daily and clopidogrel 75 mg daily for 7 days before treatment. Platelet inhibition by P2Y12 assay was not routinely tested. Intraprocedure systemic anticoagulation was instituted with heparin. Parent vessel size measurements were determined from calibrated standard digital subtraction angiography (DSA) images.
For all procedures, a triaxial system was used through an 8F femoral access, as previously described.11 This consisted of a 6F 087 Flexor Shuttle guiding sheath (Cook Medical, Bloomington, Indiana, USA) or 8F 088 Neuron MAX delivery catheter (Penumbra, Alameda, California, USA), a 5F Navien (0.058″ internal diameter, 115 cm) distal intracranial support catheter (Covidien, Mansfield, Massachusetts, USA) and a Marksman microcatheter (0.027″ internal diameter, 150 cm; Covidien, Mansfield, Massachusetts, USA).
The PED Flex was deployed under real-time visualization using native fluoroscopy (7.5 pulses/s), roadmap, and DSA (3 frames/s). Control DSA was performed immediately after deployment and at 5 and 10 min after deployment to confirm vessel wall apposition, parent vessel patency, and to rule out intraluminal thrombus. Postprocessing of the implant by balloon angioplasty was used to improve vessel wall apposition when necessary. DynaCT was done at the end of the procedure to further assess device opening and to rule out intracranial hemorrhage.
Data collection and statistical analysis
Data were collected for patient demographics, aneurysm characteristics, grade of the aortic arch, cervical ICA tortuosity (defined as a 90° turn, hairpin turn, or corkscrew loop), grade of the cavernous ICA (cICA),12 procedural details, and postprocedure length of stay (LOS). Data were presented as counts, percentages, and means. When means were presented, the SEM was used to assess sample distribution.
Results
Forty-four aneurysms in 42 patients were treated using the PED Flex between February 2015 and April 2015. These cases are presented in tables 1 and 2. Highlighted cases are presented in figures 1⇓–3.
Patient demographics, aneurysm characteristics, and access
Case properties, technical details, and length of stay
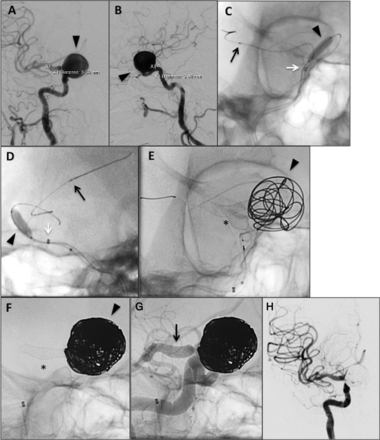
Case No 14: Pipeline embolization device (PED) Flex embolization of 13 mm left P2/3 distal posterior cerebral artery (PCA) aneurysm in a sexagenarian. (A) Anteroposterior (AP) and (B) lateral pre-embolization digital subtraction angiography (DSA) angiography of left-sided 13 mm P2/3 aneurysm. (C) 3D rotational reconstruction further demonstrating the aneurysm and involvement of the entire vessel segment. (D) With the Navien positioned in the left V4 segment, the aneurysm was crossed with a SL-10 microcatheter (black arrow=catheter tip) and Synchro-2 standard microwire. Afterwards, an intracranial exchange was used to track the Marksman microcatheter into the distal PCA. (E) Native lateral view demonstrating the Marksman (black arrow=catheter tip) in the distal left PCA. The PED Flex (3.25 mm×20 mm, black asterisk) is loaded in the Marksman. The white arrow indicates the distal end of the constrained PED Flex. (F) The PED Flex was subsequently deployed. The black asterisk indicates the implant and the black arrow the tip of the Marksman. (G) Postdeployment DSA showing contrast stasis in the aneurysm (black arrowhead). (H) DynaCT showing an open PED Flex device (white asterisk) and contrast stasis in the aneurysm (white arrowhead). (I) The patient presented on postprocedure day 15 with confusion and right-sided weakness and numbness having not complied with the antiplatelet regimen. DSA angiography demonstrated occlusion of the left PCA at the proximal end of the PED Flex device (black arrow) and (J) diffusion-weighted MRI demonstrated a left PCA-territory stroke.
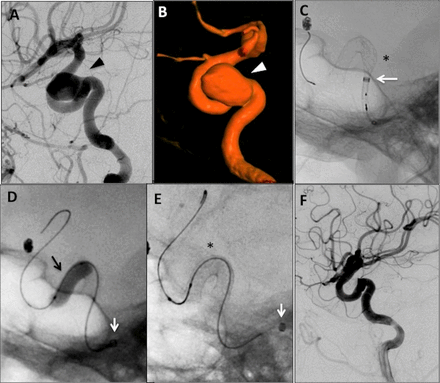
Case No 44: Pipeline embolization device (PED) Flex and coil embolization of 22 mm right ophthalmic internal carotid artery (ICA) aneurysm in a sexagenarian. (A) Anteroposterior (AP) and (B) lateral pre-embolization digital subtraction angiography (DSA) angiography of right-sided 22 mm ophthalmic ICA aneurysm (black arrowhead). (C) AP and (D) lateral native fluoroscopy images demonstrating the aneurysm being crossed. The Navien was positioned in the mid horizontal cavernous ICA (white arrow=Navien catheter tip). A Transform-compliant 4 mm×15 mm balloon (Stryker) was inflated in the aneurysm sac, and the aneurysm was crossed with a SL-10 microcatheter (black arrow=catheter tip) and Synchro-2 standard microwire. An intracranial exchange was then used to track the Marksman microcatheter into the distal right middle cerebral artery. The inflated balloon was used to prevent herniation of the catheters during these maneuvers. (E) The PED Flex (4.25 mm×25 mm, black asterisk) was then deployed from the right M1 to the cavernous ICA, and coiling was initiated when most of the PED was deployed. (F) After deployment of the PED Flex (black asterisk) proximal to the aneurysm, further coiling was done (black arrowhead). The patient had baseline right-sided blindness so mass effect on the optic nerve was not a concern. (G) Cine angiography demonstrating good vessel wall apposition of the PED Flex (black arrow). (H) Final control angiography (AP view) demonstrating occlusion of the aneurysm and normal parent vessel appearance.
Case No 9: Pipeline embolization device (PED) Flex embolization of a left-sided 12 mm cavernous internal carotid artery (ICA) aneurysm in a quinquagenarian. (A) Lateral digital subtraction angiography (DSA) and (B) 3D rotational angiography of left-sided 12 mm cavernous ICA aneurysm (arrowhead). The cavernous genu system is tortuous and classified as grade IV. (C) Lateral native fluoroscopy image demonstrating deployment of the proximal PED Flex (4.25 mm×14 mm; black asterisk) using an intra-Navien technique (white arrow=tip of Navien), which is helpful in facilitating opening and prevents twisting of the proximal device in type IV genu systems. Coils in an unrelated anterior communicating aneurysm are also visualized. (D) After PED Flex deployment, a Transform-compliant 4 mm×10 mm balloon (black arrow, Stryker) was inflated for improved vessel wall apposition of the device. (E) After angioplasty, the PED device is widely open (black asterisk). (F) Final control DSA (lateral view) demonstrating flow remodeling and normal parent vessel appearance.
Patient and aneurysm characteristics
Patient demographics and aneurysm characteristics are shown in table 1. Mean patient age was 56.6±2.0 years with a range of 24–81 years; 39 patients (93%) were women and 3 (7%) were men. Forty-one of the aneurysms (41/44, 93%) were in the anterior circulation, and 3/44 (7%) aneurysms were in the posterior circulation. The mean aneurysm size was 6.5±0.6 mm (range 3–22 mm). For aneurysm morphology, 36/44 (82%) aneurysms were saccular, 6/44 (14%) were fusiform (defined as involving >25% of the parent vessel wall), and 2/44 (5%) were dissecting. Thirty-six of the aneurysms (36/44, 82%) had no prior treatment, 4/44 (9%) were previously coiled, 3/44 (7%) were previously clipped, and 1/44 (2%) was previously treated with a PED.
Proximal vascular characteristics
Access and procedural complexity were indirectly assessed using markers such as aortic arch type, cervical ICA tortuosity, and cICA grade.12 A grade I aortic arch was present in 18/44 (41%) patients, grade II arch in 15/44 (34%) of cases, and grade III arch in 5/44 (11%) of cases. Arch imaging was not available for 6/44 (14%) of cases. Significant cervical ICA tortuosity, defined as a 90° turn, hairpin turn, or corkscrew loop, was present in 16/41 (39%) of anterior circulation cases. For the 41 anterior circulation cases, there was a large distribution of cICA grades, with 2/41 (5%) grade IA, 14/41 (34%) grade IB, 11/41 (27%) grade II, 11/41 (27%) grade III, and 3/41 (7%) grade IV.
Procedure characteristics
Details of procedure characteristics are shown in table 2. Triaxial systems were used for all procedures, with Neuron MAX used in 37/44 (84%) of cases. The Navien intracranial catheters and Marksman microcatheter were used in all procedures. A PED Flex was successfully implanted in 43/44 cases (98%). A single device was implanted in 41/43 cases (95%), and multiple devices implanted in 2/43 cases (5%), representing a mean device implantation per case of 1.07±0.05 devices. In case No 37, two attempts were made to implant the PED Flex, but these were unsuccessful. For this case, the tortuosity of the A1–2 junction prevented successful implantation of a device. The first device attempted (2.5 mm×14 mm) could not be pushed through the Marksman to the distal landing zone. The second device (3.0 mm×14 mm) made it to the distal landing zone, but the Marksman was damaged and the device could not be unsheathed. The case was subsequently aborted without device implantation.
Exchange techniques were used to cross the aneurysm in 3/44 cases (7%). This is shown in figures 1 and 2. Resheathing the device for improved positioning or opening was performed in 4/44 cases (9%). Postprocessing of the PED Flex device by balloon angioplasty to improve vessel wall apposition was performed in 10/43 cases (23%) with implantation. This is shown in figure 3. Mean heparin dose for the 44 cases was 5020±95 U (range 3000–6000 U), and mean contrast dose was 57±3 mL (range 27–90 mL). Mean fluoroscopy time (combined anteroposterior and lateral) for the cases was 32.7±2.9 min (range 13.1–105.5 min).
Length of stay and periprocedural outcomes
The mean postprocedure hospital LOS was 1.3±0.2 days (range 1–8 days). For 37 of the 44 cases (84%), the patient was discharged home on postprocedure day 1. For case No 42, the postprocedure LOS was 8 days. This patient had a postprocedure groin/anteromedial thigh hematoma requiring transfusion and associated femoral pseudoaneurysm requiring thrombin injection.
Of the 44 cases, one patient had a significant neurological complication (2%). For case No 14 (figure 1), the patient was discharged on postprocedure day 1 without complication. The patient re-presented on postprocedure day 15 with confusion, right-sided numbness, and mild to moderate weakness having not complied with the antiplatelet regimen (stopped taking aspirin and clopidogrel). MRI demonstrated a left-sided posterior cerebral artery stroke. Cerebral angiography showed occlusion of the left posterior cerebral artery at the PED with reconstitution of the distal posterior cerebral vessels via collaterals.
Discussion
We present our initial experience using the PED Flex in 44 consecutive cases of 44 aneurysms in 42 patients treated over a period of 3 months. To our knowledge, this represents the first North American series and the largest single institution experience using this next-generation PED delivery system. PED Flex was implanted successfully in 43/44 cases, representing 98% success rate. A single device was implanted in 41/43 (95%) of cases. Mean postprocedure LOS was 1.3±0.2 days, with 37/44 cases (84%) going home on postprocedure day 1. There were no intraprocedural complications. Two patients (2/44, 4.5%) had significant postprocedure complications, only one of which was neurological (1/44, 2.3%). One patient had a groin hematoma requiring transfusion and associated femoral pseudoaneurysm requiring thrombin injections. Another patient had thrombosis of the PED Flex device and associated stroke after non-compliance with the prescribed dual antiplatelet regimen.
The implant for PED Flex is the same as the original first-generation device. However, the delivery system has been redesigned with several important modifications. These have been previously described in detail.7 ,8 Briefly, the distal end of the device is no longer constrained on a capture coil and does not require torque to release. Rather, there are two polytetrafluoroethylene flaps that protect the distal braid and facilitate opening when purposely inverted.7 The proximal device is also no longer constrained, and it is now positioned on a resheathing pad. This allows resheathing of the device when up to 90% is deployed. A ‘point-of-no-return’ marker is present on the proximal delivery system to facilitate ease of use when resheathing. Furthermore, the distal tip coil is now 0.012″ with a tip angle of 55°, and the pusher wire is now a larger laser-cut hypotube with increased stiffness.
The four available articles of PED Flex cases have reported improved deployability of the implant using the newer delivery system. Martínez-Galdámez and colleagues described the use of PED Flex in six patients with six aneurysms treated at three university hospitals in Spain.7 They resheathed the device in two cases (33%), once for repositioning and the other for improved distal opening. No intraprocedural complications were reported. One patient (1/6, 17%) had a postoperative stroke, but this did not negatively affect the discharge modified Rankin Scale score. The median LOS for these six cases was 3 days. Pereira and colleagues described use of the PED Flex in 10 patients with 12 aneurysms.8 The resheath/recapture maneuver was used to reposition and improve distal opening in 5/10 (50%) cases. In one case the device could not be recaptured secondary to proximal vascular tortuosity. Balloon angioplasty was used in 1/10 (10%) cases for improved wall apposition. They reported no intraprocedural complications, but 2/10 patients (20%) had transient postprocedure neurological symptoms. Duckworth and colleagues reported a single case of PED Flex use to treat a posterior inferior cerebellar artery aneurysm.10 This is the first reported single case in the USA, and the device was used on a compassionate basis. The resheathing technique was used for improved placement of the PED Flex device. The procedure was uncomplicated. Martínez-Galdámez and colleagues reported a series of 30 patients with 30 aneurysms treated at nine academic centers in Spain.9 The resheathing technique was used for 18/39 (46%) devices in 17/30 cases (57%). In four cases resheathing was for recapture and repositioning, and in the other instances it was used to improve vessel wall apposition. This group reported an intraprocedural complication rate of 2/30 (6.7%), including occlusion of a middle cerebral artery branch in one case and in-stent thrombus formation in the other. They also reported a 30-day morbidity rate of 6.6% with two major clinical events (both anterior choroidal artery strokes).
In the series of 30 cases by Martínez-Galdámez and colleagues, the main variables analyzed were average number of devices deployed per case and the complication rate associated with recapture.9 These were used as surrogate markers of procedural complexity and risk of complication, given that complication rates with the first-generation PED have been linked to number of devices deployed and complex technical maneuvers.13 ,14 In our series, we reported implantation of 46 devices in 43 cases with successful implantation, representing a mean of 1.07±0.05 devices per case. This is less than our group’s previously published experience with first-generation PED, including a mean of 1.2±0.1 devices in our initial series of 30 anterior circulation aneurysm treatments (mix of small, large, and giant)11 and a mean of 1.1±0.05 devices per case in a subsequent series of 42 successful anterior circulation small (<10 mm) aneurysm treatments.15
Furthermore, the ability to resheath PED Flex for improved opening and for recapture/repositioning has been marked as a key modification of the newer system. This feature had been credited with improved positioning of the device and a reduction in device removal using a technique termed ‘corking’.16 In this report, we resheathed the device in 4/44 cases (9%). This is less than in the other series listed above, which used the technique in 33–57% of cases. We did not experience any intraprocedural complications during these maneuvers. Likewise, we removed the PED Flex device in only 1/44 cases (2%), which is lower than our previous reported rates of approximately 14%.11 ,15
As with the first-generation PED delivery system, robust intracranial support is recommended for deployment of the PED Flex. We used a triaxial system with the Navien catheter as our intermediate support system for all cases. We have previously described successful use of the Navien for distal17 and ultra-distal18 intracranial support during first-generation PED procedures. In the present series, robust support helped to facilitate complex PED Flex deployments in distal aneurysms (figure 1), large/near-giant aneurysms (figure 2), and in complex/tortuous cavernous genu systems (figure 3).
The new pusher/delivery wire of the PED Flex is a laser-cut hypotube and noticeably stiffer than its predecessor. The stiffer delivery wire of PED Flex helps to facilitate pushing of the constrained PED forward through the Marksman and in retracking the Marksman through the PED after deployment. The thicker pusher also helps in advancing the Navien postdeployment if needed to ‘bump’ the proximal end of the device and gain endoluminal access. While the pusher helps to advance the constrained device through tortuosity, it can also advance the Marksman catheter tip during this process. This was not seen during first-generation PED cases, and the catheter tip should be monitored during this maneuver when using PED Flex. In cases of giant aneurysms, the stiff pusher wire may be a disadvantage and promote herniation of the PED Flex delivery system into the aneurysm. Adjunct use of an intrasaccular balloon (as shown in figure 2) can provide additional support during complex maneuvers to reduce the risk of device herniation.
Published complication rates with PED Flex cases have been low and acceptable. We reported two significant postprocedure complications in 44 cases (4.5%). One complication, a groin hematoma, was unrelated to the PED Flex device. The second complication, thrombosis of the PED Flex implant, occurred in a patient non-compliant with the prescribed antiplatelet regimen. Presumably, this event would not have happened if the patient had been taking aspirin and clopidogrel as prescribed.
Conclusion
The PED Flex is a second-generation flow diverter system with significant improvements over the first-generation device. Redesign of the delivery system to improve device opening and added resheathability has resulted in improved deliverability and implantation of the PED. Our early experience demonstrates a favorable technical profile in a variety of aneurysm treatments.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Contributors GPC performed treatment procedures, collected and analyzed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. L-ML assisted in critically revision of the manuscript and analysis of data. JMC assisted with data collection and analysis. BJ helped to collect data and draft the manuscript. JH and RJT critically reviewed the important intellectual content. ALC performed treatment procedures, performed data analysis and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Competing interests ALC is a proctor for the Pipeline embolization device (Covidien, Mansfield, Massachusetts, USA) and a consultant for Covidien, a proctor for the Surpass device (Stryker Neurovascular, Fremont, California, USA) and a consultant for Stryker Neurovascular, a proctor for the FRED device (Microvention, Tustin, California, USA) and a consultant for Microvention. GPC is a consultant for Covidien and Microvention. The other authors have no conflict of interest.
Ethics approval This research was approved by the Johns Hopkins institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.