Article Text
Abstract
Objective Despite promising initial results, current knowledge regarding the use of the Low-profile Visualized Intraluminal Support (LVIS) device to treat wide-necked intracranial aneurysms is still limited. Our aim is to evaluate the feasibility, efficacy, and safety of the LVIS device in stent-assisted coiling of intracranial aneurysms.
Methods We conducted a systematic review by searching PubMed, EMBASE, and Cochrane Library for all published studies on the treatment of intracranial aneurysms with the LVIS device up to March 2016. Feasibility was evaluated by the technical success rate during the procedure, efficacy was evaluated by the rate of complete aneurysm occlusion at follow-up angiography, and safety was assessed by procedure-related morbidity and mortality.
Results A total of nine studies were included in the analysis, including 384 patients with 390 aneurysms. The overall technical success rate was 96.8% (95% CI 94.4% to 99.1%). The aneurysmal complete occlusion rate was 54.6% (95% CI 31.8% to 77.4%) on immediate control and 84.3% (95% CI 78.9% to 89.7%) at follow-up angiography. Procedural-related morbidity and mortality were 1.4% (95% CI 0.2% to 2.6%) and 0% (95% CI 0%), respectively. The thromboembolic event rate was 4.9% (95% CI 1.9% to 7.9%) and the hemorrhagic event rate was 2.1% (95% CI 0.7% to 3.5%), with 0.9% (95% CI 0% to 1.8%) experiencing neurologic hemorrhagic complications and 1.9% (95% CI 0.5% to 3.2%) experiencing non-neurologic hemorrhagic complications.
Conclusions Our systematic review suggests that endovascular treatment of intracranial aneurysms with the LVIS device is feasible, safe, and effective in the short term. However, the rate of thromboembolic complications is not negligible. Further prospective studies are needed to evaluate the long-term efficacy and safety of the LVIS device.
- Aneurysm
- Coil
- Stent
Statistics from Altmetric.com
Introduction
Endovascular treatment of intracranial aneurysms using detachable coils has been widely accepted as a safe and effective alternative to surgical clipping.1–3 However, coil embolization of wide-necked and complex-shaped aneurysms remains challenging due to the high risk of coil herniation or migration responsible for thromboembolic complications. Advanced techniques for endovascular treatment of wide-necked and complex aneurysms has been developed, such as balloon remodeling and stent-assisted coiling.4 Balloon and stent-assisted techniques allow denser coil packing by preventing coil protrusion into the parent artery. In addition, stents may induce an alteration of intra-aneurysmal hemodynamics while providing a scaffold for endothelial growth and vessel wall healing.5
A new self-expandable stent, the Low-profile Visualized Intraluminal Support (LVIS) device, is designed as an adjunct to coil embolization of wide-necked intracranial aneurysms. There is a rapidly growing literature on using the LVIS device.6–14 However, to our knowledge, no studies to date have specifically reviewed the evidence for its use. We therefore conducted a systematic review of the literature regarding this technique in the management of intracranial aneurysms. Our aim is to evaluate the feasibility, efficacy, and safety of the LVIS device in stent-assisted coil embolization of intracranial aneurysms.
Materials and methods
LVIS device description
The LVIS device is a self-expanding nickel titanium (nitinol), single-wire braid, closed-cell microstent. There are two different kinds of the device: LVIS and LVIS Junior. The LVIS stent, which is compatible with a 0.021 inch microcatheter, has a cell size of 1.0 mm and is recommended for a parent vessel size of 2.0–5.0 mm. It has four radiopaque tantalum markers on the proximal/distal ends with two radiopaque helical strands within the body of the stent. The LVIS Junior stent, which is compatible with a 0.017 inch microcatheter, has a cell size of 1.5 mm and is intended for vessels sized 2.0–3.0 mm. It has three radiopaque tantalum markers on the proximal/distal ends with three radiopaque helical strands within the body of the stent. The stent is introduced into the microcatheter and delivered to the intended site for deployment. It can be fully reconstrained after deployment of up to 80% of its length.
Search strategy
Two authors (XZ and JZ) conducted the electronic health database search using PubMed, EMBASE, and Cochrane Library for all published articles up to March 2016. The following keywords were used in combination (by using ‘AND’ and ‘OR’): ‘LVIS’, ‘LVIS Jr’, ‘Low-profile Visualized Intraluminal Support device’, ‘intracranial aneurysm (aneurysms)’, ‘brain aneurysm (aneurysms)’. In addition, a manual search of reference lists and relevant reviews was performed to identify relevant studies. For multiple series arising from the same institution or authors, we only included the most recent (usually larger) series.
Inclusion and exclusion criteria
The inclusion criteria for the reviewed studies were the following: (1) published in the English language; (2) a series of at least 10 patients with intracranial aneurysms treated by the LVIS or LVIS Junior stent; (3) documenting the rate of angiographic aneurysm occlusion; (4) data on postoperative outcomes regarding complications; and (5) reporting on duration of follow-up. Case reports, letters, review articles, technical notes, and case series with fewer than 10 patients were excluded from the study.
Literature review and data extraction
This review was prepared in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data from eligible studies were reviewed and extracted using a structured data extraction form by two authors (XZ and JZ) independently. In the case of disagreement, consensus was reached through discussion with the senior author (FX).
Outcomes
Technical success was defined as successful delivery and deployment of the study stent to the target vessel without navigation failure, stent malposition, stent migration, or incomplete stent expansion. Angiographic outcomes were extracted as immediate and follow-up aneurysmal occlusion rates, which were categorized according to the Raymond–Roy occlusion classification system: complete occlusion (Raymond I), remnant neck (Raymond II), and residual aneurysm (Raymond III). Procedure-related complications included both early and delayed, and both symptomatic and asymptomatic ischemic and hemorrhagic events. Thromboembolic events were defined as transient ischemic attack, stroke, or development of a symptomatic thrombus during the procedure. Recanalization referred to any further filling of the aneurysm neck or sac during follow-up. Clinical outcome was assessed using the modified Rankin Scale (mRS). Morbidity and mortality were defined as a deterioration of >0 on the mRS and any death related to the treatment.2
Statistical analysis
All the studies were non-comparative. We estimated the cumulative incidence (event rate) and 95% CI for each outcome. Event rates were pooled across studies using random-effects meta-analysis.15 Heterogeneity across studies was evaluated by means of the I2 statistic.16 Publication bias was unable to be assessed due to the non-comparative nature of the studies. This meta-analysis was performed using Comprehensive Meta-Analysis V.2.0 (http://www.meta-analysis.com).
Results
Study selection
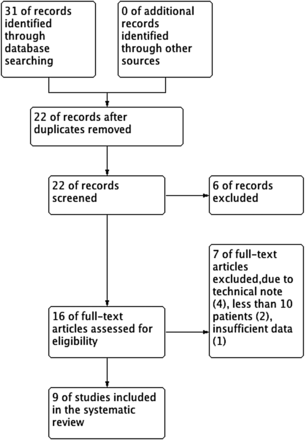
A flow diagram showing the process of identifying relevant articles is given in figure 1. The electronic database search yielded a total of 31 potentially relevant articles, of which nine met our inclusion criteria. No new articles were found by a manual search of the references. As a result, nine studies were included in this analysis.
Flow diagram for the selection of eligible studies.
Characteristics of patients and aneurysms
Of the nine included studies, five were retrospective studies and four were multicenter prospective studies. All these studies were non-comparative and published between 2014 and 2016. The studies were performed in China (n=3), Germany (n=2), Belgium (n=1), Korea (n=1), the USA (n=1), and Poland (n=1). A total of 384 patients with 390 aneurysms were included. Almost all of the saccular aneurysms had a wide neck (neck width >4 mm or dome to neck ratio <2). Of 390 treated aneurysms, 40 (10.3%) were ruptured. Five studies reported using the LVIS Junior stent only, one study reported using the LVIS stent only, and three studies reported using both the LVIS and LVIS Junior stents. The main locations of the aneurysms were the anterior communicating artery, middle cerebral artery, internal carotid artery (ICA), and basilar artery. Most patients were followed up and measured by angiographic and clinical assessment. All included studies reported their antiplatelet therapy strategy, mainly dual antiplatelet therapy with aspirin and clopidogrel before the procedure. Table 1 summarizes the baseline characteristics of the included studies, patients, and aneurysms.
Baseline data of included studies
Study outcomes
The overall technical success rate was 96.8% (95% CI 94.4% to 99.1%). Complete occlusion (Raymond I) rate was 54.6% (95% CI 31.8% to 77.4%) on immediate control and 84.3% (95% CI 78.9% to 89.7%) at follow-up angiography (table 2), remnant neck (Raymond II) rate was 32.9% (95% CI 18.6% to 47.2%) on immediate control and 8.8% (95% CI 5.4% to 12.2%) at follow-up angiography, and residual aneurysm (Raymond III) rate was 16.3% (95% CI 6.8% to 25.7%) on immediate control and 4.3% (95% CI 1.2% to 7.5%) at follow-up angiography. The recanalization rate was 2.5% (95% CI 0.5% to 4.6%).
Outcomes
The procedure-related complication rate was 6.5% (95% CI 4.1% to 9.0%) and the procedure-related morbidity rate was 1.4% (95% CI 0.2% to 2.6%). The thromboembolic event rate was 4.9% (95% CI 1.9% to 7.9%), with 2.4% (95% CI 0.9% to 3.9%) experiencing symptomatic thromboembolic events and 1.4% (95% CI 0.2% to 2.5%) experiencing in-stent thrombosis. The hemorrhagic event rate was 2.1% (95% CI 0.7% to 3.5%), with 0.9% (95% CI 0% to 1.8%) experiencing neurologic hemorrhagic complications and 1.9% (95% CI 0.5% to 3.2%) experiencing non-neurologic hemorrhagic complications. However, no mortality was reported in all the included studies (table 3).
Complications
Heterogeneity and publication bias
Analysis of technical success, recanalization, and complications has a low heterogeneity. The immediate and follow-up occlusion rates were associated with significant heterogeneity (I2). However, due to insufficient information provided in the included studies, subgroup analysis and further analysis of possible causes for the significant heterogeneity were unable to be conducted. It was not possible to measure publication bias due to the small number and non-comparative nature of the included studies.
Discussion
Despite initial promising results, the current knowledge regarding the use of the LVIS device to treat intracranial aneurysms is still quite limited. Our study represents a systematic review of the current literature on stent-assisted coiling of wide-necked intracranial aneurysms using the LVIS device. Overall, our analysis demonstrates high rates of complete occlusion at short-term follow-up for aneurysms treated with the LVIS device. However, we also demonstrate that thromboembolic complications are not negligible, with a rate of 4.9%. In-stent thrombus is uncommon, but most of these cases are asymptomatic and can be treated successfully with glycoprotein IIb/IIIa inhibitors. Procedure-related morbidity and mortality occurred in only 1.4% and 0% of patients, respectively. These findings suggest that stent-assisted coiling with the LVIS device is a safe and effective treatment strategy for intracranial aneurysms.
Our study demonstrated that the LVIS device is feasible with a high technical success rate of 96.8%. Failure of the stent to expand adequately was the main cause. Moreover, segmentally incomplete expansion occurred most often in the use of the LVIS stent. Cho et al7 observed incomplete stent expansion in five cases in whom the LVIS stent was deployed from the distal ICA to the cavernous ICA. They suggested that the LVIS stent might fold or twist due to the tortuous anatomy and acute curve of the carotid siphon. Compared with the LVIS standard stent, the LVIS Junior stent uses the smallest available delivery system (0.017 inch microcatheter). The advantage of this stent is that it allows excellent tracking and precise advancement in tortuous and distal vessels. In addition, new generations of the LVIS Junior stent increase more radial force and decrease flared ends. All of these new modifications facilitate the stent to open fully and appose the parent vessel wall. Thus, incomplete stent expansion was not frequently associated with the LVIS Junior stent. Failure to deliver the stent to the target location or stent migration was also relatively rare in our study.
Although immediate angiographic results in the literature review demonstrated relatively lower rates of complete occlusion, progression to total occlusion was quite impressive with a high rate of complete occlusion of 84.3% at 4.2–6 months. The results of LVIS stent-assisted coiling of intracranial aneurysms are comparable to those reported for the Neuroform and Enterprise stents. King et al17 reported the largest literature review comparing stent-assisted coiling between Neuroform and Enterprise. Initial and final complete occlusion was seen in 52.7% and 61.1%, respectively, of patients treated with Neuroform. In contrast, the Enterprise system was associated with higher complete occlusion (74.7%) at follow-up. Recently, Cho et al7 reported a complete obliteration rate of 92.6% at 6-month follow-up in a series of 55 patients treated with the LVIS device. However, the patient cohort remained relatively small and follow-up was still short term. Further studies with larger patient cohorts and long-term follow-up are required to evaluate the efficacy of the LVIS device.
The overall procedure-related complication rate is 6.5%, which is comparable with other studies on different intracranial stents. Gory reported a relatively high rate of overall periprocedural complications with the Solitaire device (12.7%) compared with Neuroform (12.1%) and Enterprise (10.0%).18–20 Only two intracranial hemorrhages occurred in our study and there were six cases of groin hematomas that did not require any surgical intervention, so the rate of hemorrhagic complications is quite low. However, the risk of thromboembolic complications is relatively higher. As shown in our study, the occurrence of thromboembolic events is 4.9%. King et al17 reported similar rates of thromboembolic events with Neuroform (6.7%) and Enterprise (5.9%) stents. Thromboembolic events are major complications of stent-assisted coiling of intracranial aneurysms. Incomplete stent expansion is a common cause, especially when wire-braided stents are used. However, the LVIS stent offers better visualization, improved wall apposition, and less stent bending than the other devices. Thus, the precise means of thromboembolic events remain elusive.
Thromboembolic complications include symptomatic thromboembolic events and asymptomatic thrombus. Luo et al21 showed that symptomatic thromboembolic complications were more frequent with the Leo stent (12%), and Kadkhodayan et al22 reported a higher rate of symptomatic thromboembolic events associated with the Enterprise stent than with the Neuroform stent (8.7% vs 1.4%). Concerning the Solitaire stent, Gory et al19 reported a symptomatic thromboembolic complication rate of 7.9%. In our study the rate of symptomatic thromboembolic complications was quite low (2.4%). In-stent thrombus was uncommon in our systematic review, with a rate of 1.4%. Most cases of in-stent thrombus were asymptomatic and only two cases experienced symptomatic thromboembolic complications. All these cases were successfully treated with intravenous administration of glycoprotein IIb/IIIa inhibitors. Previous studies have strongly suggested that insufficient platelet inhibition was associated with an increased risk of thrombus formation and embolic complications. Dual antiplatelet therapy with aspirin and clopidogrel before the procedure was used in all included studies. However, a platelet function test was performed in only three studies. Moreover, insufficient platelet inhibition was confirmed in one patient who suffered in-stent thrombus. Delayed thrombotic events might be due to non-compliance with antiplatelet medication. These data suggest that, besides dual antiplatelet therapy, routine platelet function testing is recommended before endovascular procedures.
Procedure-related morbidity (1.4%) and mortality (0%) are low and are comparable with previously published data for other intracranial stents.18 ,19 ,23 Mocco et al23 reported a large multicenter series of Enterprise stent-assisted coiling of intracranial aneurysms. The overall procedure-related morbidity was 6%; procedure-related permanent morbidity and mortality were 2.8% and 2%, respectively. Gentric et al18 reported a prospective multicentre study of the Neuroform stent system and found permanent neurologic morbidity and mortality rates of 1.9% and 0%, respectively. Gory et al19 reported a prospective multicentre study of the Solitaire AB stent; mortality related to the procedure was 0% and permanent morbidity was 1.6%. Our results demonstrated that the use of the LVIS device in stent-assisted coiling of intracranial aneurysms is acceptable.
This systematic review has several limitations. First, five of nine included studies were retrospective and none of them was randomized. Most studies had a small sample size and did not include control groups. Second, the potential for observer bias in the reporting of outcomes exists in our study. Third, conflict of interest is one potential source of bias in clinical studies. Financial relationships were reported in two studies, including consultation and research support. Of the remaining five studies, there is no conflict of interest. Finally, subgroup analysis of aneurysms size, status (ruptured or unruptured), and locations were unable to be conducted due to insufficient data.
Conclusions
Our study suggests that endovascular treatment of intracranial aneurysms with the LVIS device is feasible, safe, and effective in the short term. However, the rate of thromboembolic complications is not negligible. In addition, there are no available studies comparing stent-assisted coiling with the LVIS device and other intracranial stents. Further prospective studies are needed to evaluate the long-term efficacy and safety of the LVIS device.
References
Footnotes
XZ and JZ contributed equally.
Contributors Conception and design: FX. Analysis and interpretation of the data: XZ and JZ. Drafted the article: FX. Critically revised the article: FX and NCB. Reviewed submitted version of manuscript: FX and NCB. Approved the final version of the manuscript on behalf of all authors: FX and NCB. Statistical analysis: FX and JZ. Administrative/technical/material support: HG.
Competing interests None declared.
Ethics approval Ethics approval was obtained from Huashan Hospital, Fudan University.
Provenance and peer review Not commissioned; externally peer reviewed.