Article Text
Abstract
Background It has been amply demonstrated that endovascular procedures can be successful treatment for stroke, both in terms of revascularization and clinical outcome. There is not, however, a published comparison of any histological or ultrastructural damage to the vessels that may be caused by a direct aspiration first pass technique (ADAPT) or stent retrievers (SR) used in these procedures. This study analyses and compares acute damage to the arterial wall caused by ADAPT or SR.
Material and methods Damage to the walls of swine extracranial arteries was evaluated after ADAPT with the Penumbra system or thrombectomy with an SR (Solitaire 6×30). The procedures were performed after injecting thrombi into the selected arteries (arteries with diameters similar to those of the human internal carotid artery and first segment of the middle cerebral artery). After the procedures, the animal was euthanized and 12 arterial samples were obtained for analysis by optical and electronic microscopy.
Results Tissue samples from the vessels treated with SR showed almost complete loss of endothelium, thickening of the internal elastic lamina, and degeneration of the elastic fibers of the bordering lamina media and adventitia. In contrast, tissue samples of the vessels treated with ADAPT had a clear integral internal elastic lamina and uninterrupted endothelial lining, although cell alignment was altered and there were surface lacerations due to manipulation of the samples.
Conclusions Both techniques caused acute damage to the vessel walls, however, thrombectomy with SR appeared to be more harmful to all layers of the arterial wall, particularly the endothelium.
- Stroke
- Thrombectomy
- Stent
- Vessel Wall
- Artery
Statistics from Altmetric.com
Introduction
The success, in terms of revascularization and clinical outcomes, of endovascular procedures for the treatment of stroke has been amply demonstrated.1–5 These procedures have evolved over the years, with initially greater attention given to the development of stent retrievers (SR) (eg, the Solitaire (Medtronic, Irvine, California, USA) in 2009, the Trevo (Stryker, Kalamazoo, Michigan, USA) in 2010, and the Revive (Codman, Raynham, Massachusetts, USA) in 2011).6 Machi et al7 recently analyzed the various mechanical properties and efficiency of the SR available on the market. The introduction of ADAPT (a direct aspiration first pass technique), in 2014,8 associated at first with use of the Penumbra aspiration system (Alameda, California, USA) and the new large bore aspiration catheters (ACE64, Penumbra Inc/SOFIA, MicroVention Inc/AXS Catalyst Distal Catheter, Stryker) enabled diversification and, in some cases, improvement of the technique; in fact, Turk et al8 ,9 and Romano et al10 demonstrated the notable revascularizing capacity and good clinical outcome of this technique and the first results of the use of larger bore aspiration catheter were published recently.11
Although revascularizing capacity, clinical outcome, and cost efficiency of ADAPT and SR have been compared,12 there are no published comparisons of any arterial damage that may be caused by the devices used in the two techniques.
The aims of this study were to assess the histological and ultrastructural changes occurring in the arterial wall in the acute phase following endovascular treatment with ADAPT or SR, and compare the findings in order to determine which is the least harmful technique.
Materials and methods
We performed the endovascular procedures in the Animal Laboratory of the University of Bordeaux, France, with a monoplane Siemens angiography system, using the pig as an animal model (swine model 1). All procedures were conducted in accordance with international guidelines and were approved by the responsible local authorities.
The procedures were carried out with the pig under general anesthesia. No heparin bolus or other thrombolytic drugs were administered. Vital parameters, such as arterial blood pressure, heart rate, and carbon dioxide levels, were recorded continuously. Within 20 min after the end of the last retrieval/aspiration, the animal was euthanized.
The protocol was principally designed to assess the degree and type of vascular damage. Recanalization rate, vasospasm, arterial perforation, and distal embolism were also reported (table 1).
Additional information
Clot preparation
Twenty-four hours before the procedures, whole blood samples were collected into standard tubes without sodium citrate solution to prepare the artificial clot. Swine blood (60 mL), incubated at 4°C for 24 hours, was used. The solid component was separated from the plasma, broken into several pieces, and subsequently stored at 37°C until the time of the procedure.
Endovascular procedure
Following surgical exposure of the neck vessels and femoral artery, an 8 Fr guiding catheter (Neuron MAX.88, Penumbra, Alameda, California, USA) was introduced and continuously flushed with physiological saline. A diagnostic angiogram was performed to select, on the basis of their diameter, common carotid arteries as models of the internal carotid artery (ICA) and middle segment of the superficial cervical arteries for the middle cerebral artery (M1) model. Mechanical thrombectomy was carried out with both ADAPT and SR in these arteries (figure 1). A surgical clip was placed close to each artery as a landmark, and the vessels to be used as controls were identified.
Study protocol. In the first stage, we selected the arteries, on the basis of their diameter, in which to perform the mechanical thrombectomy with ADAPT or SR. In the second stage, we performed the procedures and chose the arterial segments to remove and study. In the third stage, we subjected the selected segments to optical microscopy (OM) and electron microscopy (EM). ADAPT, a direct aspiration first pass technique; ICA, internal carotid artery; SR, stent retriever.
We decided, according to convention, to perform the thromboaspiration on the right and thrombectomy with SR on the left; for selective thromboembolisation, the preformed clot was injected directly into the vessel through the 8 Fr guiding catheter.
Thromboaspiration was performed in the arterial models ICAADAPT and M1ADAPT (figure 2) with the Neuron MAX delivery catheter, an ACE64 aspiration catheter for ICAADAPT and for M1ADAPT and the Penumbra aspiration pump.
ADAPT. (A) Obstructed ICAADAPT. (B) Roadmap. (C) Aspiration for 3′. (D) Re-opened ICAADAPT. ADAPT, a direct aspiration first pass technique; ICA, internal carotid artery. See text for description of the arterial models.
Mechanical thrombectomy was performed in the arterial models ICASR and M1SR with the Neuron MAX delivery catheter, a PXSlim microcatheter (Penumbra, Alameda, California, USA), a Syncro 2 microguide (Stryker), and a Solitaire FR 6×30 mm stent (figures 1, 3).
Solitaire opened in M1SR. SR, stent retriever.
A single attempt was made for each of the procedures; the retrieval attempts were conducted according to the manufacturers' instructions and clinical practice.
Histological analysis
At the end of the procedures we selected the arterial segments to take for analysis by optical and electron microscopy, according to the criteria reported in figure 1: ICAADAPT, the tract of the right ICA between the distal tip of the aspiration catheter (which was in contact with the clot) and the delivery catheter; M1ADAPT, the main branch of the right thyrocervical trunk, between the distal tip of the aspiration catheter and the origin of the vessel; ICACONTROL, the tract of the right ICA distal to the clot; M1CONTROL, the main branch of the right thyrocervical trunk, distal to the clot; ICASR and M1SR, respectively, the left ICA and the main branch of the left thyrocervical trunk, from the point at which the stent was released to the distal tip of the delivery catheter.
Two tissue samples were taken from each artery and embedded in paraffin (PFA 4%), using the LEICA TP1020 processor (R02-HIS-14). The paraffin blocks obtained were then sectioned with a LEICA RM2255 microtome (section width, 6 μm). The sections were stained with hematoxylin–erythrosin–saffron according to the procedures used at CIC-IT (R02- HIS-04); additional staining with orcein, according to the protocol used at CIC-IT (R02-HIS-05), was performed to visualize the elastic fibers and, in particular, the internal elastic lamina. The histological analysis was performed using a Nikon Eclipse microscope and image analysis software, NIS Elements D V.3.12.
Both the histological analysis and the scanning electron microscopy (MEB FEI QUANTA 200) were performed in Bordeaux Imaging Centre: these analysis were reviewed by an indipendent pathologsit.
Results
Optical and electron microscopy of the tissue samples revealed changes to the arterial walls (table 2).
Arterial wall alterations
ICACONTROL
With optical microscopy, areas of discontinuous endothelium could be seen, with a clear internal elastic lamina (figure 4C), interrupted at some points (probably due to postoperative handling). Electron microscopy revealed areas with and without endothelial cells and the presence of numerous red blood cells. The endothelial cells appeared morphologically abnormal.
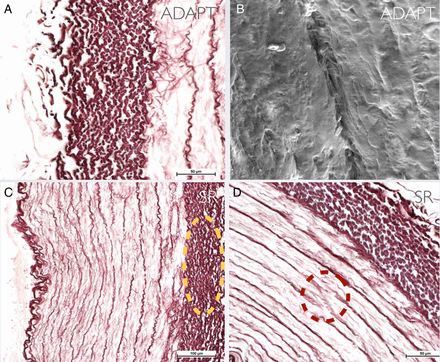
Structure of the wall of the control segment of the artery. (A) Diagram. (B) Endothelial cells stained with H&E. (C) Internal elastic lamina stained with orcein. (D) External elastic lamina stained with orcein.
M1CONTROL
On optical microscopy, the endothelial lining was present throughout the vessel (figure 4B) and the internal elastic lamina was clear and undamaged. Electron microscopy revealed areas without endothelial cells and other areas with a substantial endothelial lining.
ICAADAPT
Optical microscopy of both samples showed a clear, integral internal elastic lamina, unbroken endothelium lining the whole vessel (figure 5A), and degeneration of the elastic fibers between the tunica media and the adventitia (figure 6A). Scanning electron microscopy revealed ‘scratches’ (figure 6B): these lacerations of the tissue could be due to the aspiration system or, more probably, weakening of the tissue during its manipulation.
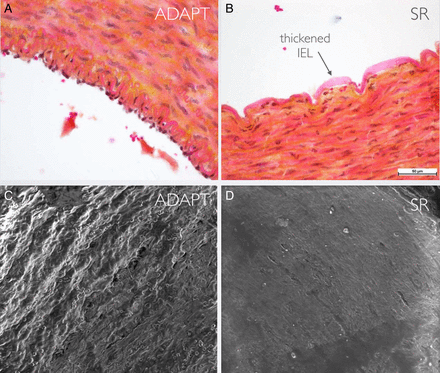
Comparison of endothelial damage assessed by optical and electron microscopy. (A, C) Segment of artery treated with ADAPT in which the endothelial layer is preserved. (B, D) Segment of artery treated with SR in which the endothelial lining is completely lost and there is reactive thickening of the IEL. ADAPT, a direct aspiration first pass technique; IEL, internal elastic lamina; SR, stent retriever.
Further changes to the external layers of the arterial wall. (A) Alteration of the bordering tunica media/adventitia in the ICAADAPT sample. (B) Parietal ‘scratches’ seen by electron microscopy in the ICAADAPT sample. (C) Alteration of the bordering tunica media/adventitia in the ICASR sample. (D) Rupture of elastic fibers in the ICASR sample. ADAPT, a direct aspiration first pass technique; ICA, internal carotid artery; SR, stent retriever. See text for description of the arterial models.
M1ADAPT
The internal elastic lamina appeared to be substantially undamaged; only rare endothelial cells were visible on the arterial surface. Paradoxically, electron microscopy showed a considerable endothelial lining, but with altered cell alignment. Numerous red blood cells were visible. It should be noted that the segment of artery studied by electron microscopy was different from that studied by optical microscopy which could explain the different findings.
ICASR
The endothelial lining was almost completely absent (only rare endothelial cells could be seen) and the internal elastic lamina was undamaged, but thickened (figure 5B). This finding was confirmed by electron microscopy, which revealed a few endothelial cells incorporated in a fibrin matrix (figure 5D). Degeneration of the elastic fibers of the bordering tunica media/adventitia was also observed (figure 6C). Furthermore, in fragment Aa2 there was distension of some of the elastic fibers of the tunica media at the thinnest area of the arterial wall (orcein staining) (figure 4D).
M1SR
The internal elastic lamina of the sample was damaged and there were only rare endothelial cells; furthermore, there were alterations of the elastic fibers of the tunica media. Electron microscopy showed areas lacking endothelial cells, confirming the histological analysis.
Discussion
It has recently been demonstrated that mechanical thrombectomy can resolve large vessel occlusion, providing an alternative and synergistic method for restoring blood flow in cerebral vessels.1–5
There are currently a variety of devices available to neurointerventionists to perform this type of treatment; indeed, over the years various different types of SR have been designed and recently a new technique, ADAPT, using large bore catheters, has been developed.6 ,8 Both methods have been shown to be effective with regards to revascularization and improving the patients' clinical outcomes.10 However, little is known about the exact mechanism of arterial injury caused by these procedures, partly because of limited angiographic/histological data from the treated arteries.
There have been several studies of the damage caused by SR in animal models:13–15 Nogueira et al13 were the first to demonstrate in an animal model that SR cause damage to the vessel wall, while Arai et al14 evaluated which of the most commonly used SR, the Solitaire and the Trevo, causes least damage to the vessel wall. For this purpose, besides histological studies, imaging techniques were applied after mechanical thrombectomy with SR. Based on angiographic follow-up, Kurre et al16 evaluated the decrease in arterial diameter at the level at which the thrombectomy was performed, while Power et al,17 using MRI, observed arterial wall thickening in the part of the vessel treated. However, to our knowledge, no study has compared the histopathological and ultrastructural effects induced by SR and ADAPT. The spread of these techniques in standard clinical practice makes it important to understand what damage the devices used can cause to the vessel wall.
The use of extracranial swine arteries to assess the efficacy of mechanical thrombectomy is well established and was described by Yuki et al.18 The normal histological characteristics of porcine extracranial arteries are well known and offer the possibility of evaluating pathological lesions.18 On this background, we designed a protocol for use of swine to assess the histological and ultrastructural damage to vessels treated with mechanical thrombectomy, comparing the two most widely used techniques, SR and ADAPT. The protocol was principally designed to assess the degree and type of vascular damage. Recanalization rate, vasospasm, arterial perforation, and distal embolism were also reported (table 1).
It was seen that both techniques cause alterations to the structure of the vessel wall (figure 4 and table 2); in detail, there was almost total denudation of the endothelium together with thickening of the internal elastic lamina in the vessels treated with SR (ICASR and M1SR) (figure 5); on electron microscopy only a few endothelial cells could be seen incorporated in a fibrin matrix and associated with degeneration of the elastic fibers of the bordering tunica media/adventitia (figure 6). In contrast, in the vessels treated with ADAPT (ICAADAPT and M1ADAPT), optical microscopy revealed a clear undamaged elastic lamina in both samples and a continuous endothelium lining the vessels, although the cell alignment of the M1ADAPT segment was altered (figure 5). There was some degeneration of the elastic fibers between the tunica media and the adventitia, albeit to a lesser extent than that found following SR treatment (figure 6). Scanning electron microscopy of ICAADAPT revealed the presence of ‘scratches’: these tissue lacerations seem to be due to the aspiration system or, more probably, weakening of the tissue during handling (figure 6).
Several factors may explain these lesions; firstly, unlike the aspiration technique which applies an aspiration force to the proximal base of the thrombus, the SR used in thrombectomy capture the clot by exerting a continuous radial force against the vessel wall, which may injure the endothelium.
In a recent study by Machì et al,7 in which various SR were compared, it was seen that the radial force of the devices varied greatly. In detail, the Trevo-PV 4–20 (0.01480 N/mm) and the Eric 3–20 (0.01850 N/mm) exerted the greatest radial force, while the Solitaire 6-30 produced a radial force of 0.00351 N/mm. Despite the Solitaire exerting less radial force than the mean force produced by the other devices, we observed numerous histological and ultrastructural alterations following the use of this SR (table 2). In particular, it emerged that the damage caused by the SR was deeper than that caused by ADAPT: there were more changes to the tunica media/adventitia and rupture of the elastic fibers in the ICASR specimen. Interestingly, previous research showed that the extent and depth of a vascular lesion may be contributing factors in promoting early atherosclerotic and accelerated hyperplastic intimal and medial changes;16 indeed, large areas of endothelial denudation without substantial medial trauma caused only mild intimal thickening, whereas focal endothelial denudation with substantial medial trauma produced marked delayed intimal thickening.19
The study by Machì et al7 also compared clot removal capacity, showing that larger devices (6 mm in diameter) have a better clot removal performance and increase the probability of capturing the clot. This is the reason why the size of the stent is increased in clinical practice in some cases; precisely in order to reproduce routine practice faithfully, we decided to use this size (6–30 mm) of SR for our study. The histological and ultrastructural findings in the ICASR and M1SR segments were not substantially different; we can, therefore, consider that the size of the stent in relation to the diameter of the vessels does not influence the severity of the vascular damage. However, further studies on a larger number of samples are necessary to confirm these observations.
As mentioned, the differences in histological and ultrastructural damage are probably due to the different mechanisms of clot removal. In detail, ADAPT, unlike SR, acts on the clot, explaining the lesser damage to the vessel wall. However, it is precisely this mechanism of action that could account for the slight increase in embolization to new territories that has been found with ADAPT.10
Given these results, ADAPT seems to be less traumatic to the vessel wall, and in particular to the endothelial layer. However, in this study, we only analyzed the acute damage to the vessel wall after a single attempt at clot removal. Further studies should clarify whether the vascular damage is directly proportional to the number of passages with the SR or ADAPT.
Our study does have some other limitations. Firstly, although the swine model is fairly realistic,18 the arteries of the human intracranial circulation have a much thinner adventitia and do not have an elastic lamina between this and the muscle layer;20 additionally, human intracranial arteries are known to have thinner vessel walls and a lower wall:lumen ratio than the small arteries in other parts of the human body18 ,20 so it can be expected that damage to the vessel wall is greater in normal clinical practice. Further investigation with a larger number of specimens is required to better understand the cause and effect relationship between thrombectomy maneuvers and tissue reaction/damage observed in treated arteries. Moreover, in this study only acute damage to the vessel wall was evaluated; in the future it would be interesting to evaluate both the evolution of the parietal lesions in the subacute phase and in the long term, and whether the damage influences patients' outcomes in any way. Finally, given that a single swine model was used, it was not possible to obtain a sufficiently large number of samples to be able to perform a statistical analysis.
Conclusion
In this animal model we found that both ADAPT and SR devices caused various vascular injuries. However, it would appear that SR produce much more aggressive damage to the endothelium and tunica media. Further studies are essential in order to confirm these initial findings.
Acknowledgments
The authors would like to thank Bordeaux Imaging Centre for the histological and the scanning electron microscopy analysis, and the Bordeaux University Animal Lab for the technical support.
References
Footnotes
Contributors Conception and design: SP. Acquisition of the data: SP and FD. Analysis and interpretation of the data: SP, FD, and JB. Drafting the article: SP. Critically revising the article: JB and PM. Reviewed submitted version of manuscript: all authors. Study supervision: JB. Histological analysis and scanning electron microscopy (MEB FEI QUANTA 200) were performed by an independent center (Bordeaux Imaging Centre).
Funding This work was partially supported by Penumbra.
Competing interests SP and JB are educational consultant for Penumbra.
Provenance and peer review Not commissioned; externally peer reviewed.