Article Text
Abstract
Objective To conduct a meta-analysis of randomized trials to comprehensively compare the effect of endovascular thrombectomy (EVT) versus intravenous thrombolysis (IVT) plus EVT on functional independence (modified Rankin Scale (mRS) 0–2) after acute ischemic stroke due to large vessel occlusions (AIS-LVO).
Methods We searched Pubmed, EMBASE, CENTRAL, and clinicaltrials.gov from January 2000 to February 2021 and abstracts presented at the International Stroke Conference in March 2021 to identify trials comparing EVT alone versus IVT plus EVT in AIS-LVO. Five non-inferiority margins established in the literature were assessed: −15%, −10%, −6.5%, −5%, and −1.3% for the risk difference for functional independence at 90 days.
Results Four trials met the selection criteria, enrolling 1633 individuals, with 817 participants randomly assigned to EVT alone and 816 to IVT plus EVT. Crude cumulative rates of 90-day functional independence were 46.0% with EVT alone versus 45.5% with IVT plus EVT. Pooled results showed the risk difference of functional independence was 1% (95% CI −4% to 5%) between EVT alone versus IVT plus EVT. The lower 95% CI bound of −4% fell within the non-inferiority margins of −15%, −10%, −6.5%, and −5%, but not −1.3%. Pooled results also showed the risk difference between EVT alone versus IVT plus EVT was 1% (95% CI −3% to 5%) for mRS 0–1, and 1% (95% CI −1% to 3%) for symptomatic intracranial hemorrhage.
Conclusions This meta-analysis suggests that EVT alone is non-inferior to IVT plus EVT for several, but not the most stringent, non-inferiority margins.
- thrombectomy
- thrombolysis
- stroke
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
INTRODUCTION
Mechanical endovascular thrombectomy (EVT) works well at reperfusing occluded large cerebral vessels and has become the standard reperfusion therapy for acute ischemic stroke due to large vessel occlusions (AIS-LVO) in the anterior circulation.1 For AIS-LVO within 4.5 hours, administration of intravenous thrombolysis (IVT) before EVT in IVT-eligible patients has been the standard approach based on its universal use in the pivotal EVT randomized controlled trials (RCTs).2 However, the fact that IVT has limited efficacy in proximal vessel occlusions raises an open, important question about whether pursuing EVT alone could be a viable alternative to the standard strategy of initiating IVT prior to EVT.3–5
Using bridging IVT before EVT may confer several advantages. Although IVT alone achieves reperfusion prior to EVT infrequently, when it does the duration of brain ischemia is reduced.4 Intravenous (IV) thrombolytics may ‘condition’ the target thrombus making it more responsive to EVT and may dissolve distal thrombi arising from clot fragmentation during retrieval.5 When navigating a catheter to the target occlusion turns out to be unworkable, IVT may be the only reperfusion therapy a patient could have received.
Conversely, bridging IVT before EVT may also yield several disadvantages. The time taken to initiate IVT might delay the start of EVT. IVT may cause the target thrombus to partially lyse and migrate to a more distal arterial segment, beyond the reach of EVT.6 IVT may increase the frequency of hemorrhagic transformation.7 When a patient has severe cervical or intracranial atherosclerosis in addition to thrombus, angioplasty and stenting may be needed and use of IVT will preclude employment of double antiplatelet therapy for stent protection during the first 24 hours post-procedure.
Direct EVT would be useful in clinical practice not only if it was superior, but also if it was only as good as bridging IVT and EVT, as the EVT alone strategy is easier to implement and avoids the cost of IV lytic agents. Accordingly, RCTs have been undertaken using non-inferiority designs to compare direct EVT and bridging IVT plus EVT. As individual trials may be underpowered to fully address this clinical issue and have variability in aspects of design (eg, dose of alteplase) and enrolled populations (eg, Asians vs non-Asians), a non-inferiority meta-analysis of completed trials is desirable to synthesize all randomized evidence.
Methods
This study was a systematic review and meta-analysis of RCTs and so did not require IRB or ethics committee approval. This study was performed according to the recommendations of the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement.8
Data sources and searches
We searched Pubmed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and the clinical trial registry maintained at clinicaltrials.gov from January 1, 2000 to February 1, 2021 with the terms: thrombectomy or endovascular treatment or revascularization or endovascular or reperfusion or mechanical thrombectomy and intravenous thrombolysis or alteplase and acute ischemic stroke or stroke or occlusion or acute cerebral infarction or large vessel. We restricted our search to human and clinical trials. There were no language restrictions. We also searched abstracts presented at the International Stroke Conference in March 2021, as well as reviewing the Introduction and Discussion sections of retrieved trials and relevant review articles to identify additional trials.
Study selection
Criteria for inclusion of a study were: (1) the study design was an RCT; (2) patients had AIS-LVO; (3) trials compared EVT alone versus IVT before EVT; and (4) trials reported an endpoint of functional independence, defined as modified Rankin Scale (mRS) 0–2, at 90 days. Studies were excluded if (1) the study design was case reports, case-control studies, cohort studies or (2) post hoc analysis of an RCT that was originally not designed to compare EVT alone versus IVT before EVT.
Outcomes
The primary efficacy outcome was functional independence (mRS 0–2) at 90 days. Secondary efficacy outcomes were freedom from disability (mRS 0–1) at 90 days and successful reperfusion at final angiogram, defined as expanded Thrombolysis in Cerebral Infarction (eTICI) scale score ≥2 b.9 Safety outcomes were any intracranial hemorrhage, symptomatic intracranial hemorrhage, and all-cause mortality by 90 days.
Data abstraction
One investigator (CHL) abstracted the data and another investigator (ML) reviewed the abstracted data. Any discrepant judgments were resolved by joint discussion. We abstracted data by treatment group about baseline characteristics including age, sex, medical history, workflow times, and patient number in each group and about each of the efficacy and safety outcomes.
Quality assessment
The risk of bias (eg, sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other issues) for each trial was assessed in accordance with the Cochrane risk of bias tool 2.0. The risk of bias was rated as low, unclear, or high according to established criteria.10
Statistical analysis
Data were analyzed in accordance with the intention-to-treat principle. Risk difference with 95% confidence intervals (CIs) was used as a measure of the effect of EVT alone versus IVT before EVT on the primary and secondary endpoints. Findings were assessed within a framework of five non-inferiority margins established in the acute stroke literature for dichotomized mRS outcomes: −15%, −10%, −6.5%, −5%, and −1.3%. The three least stringent margins were employed in completed RCTs or the trials being analyzed: −15% in a prior trial comparing two EVT techniques,11 -10% in one of the analyzed trials,12 and −6.5% in a prior trial comparing two doses of IV alteplase.13 The two more stringent margins were found in expert surveys as the minimally clinically important difference for mRS outcomes: −5% in a survey not correcting for anchoring bias and −1.3% in a survey correcting for anchoring bias.14–16 We computed a random-effect estimate based on the Mantel–Haenszel method when two or more studies provided sufficient data for a given outcome.
Heterogeneity was assessed by P value of chi-square statistics and I2, which describes the percentage of variability in the estimates that is due to heterogeneity rather than chance. We considered study-level estimates to be heterogeneous if the I2 statistic was greater than 50%. A two-tailed P<0.05 was considered statistically significant in all analyses. The Cochrane Collaboration’s Review Manager Software Package (RevMan 5.3) was used for this meta-analysis.
Results
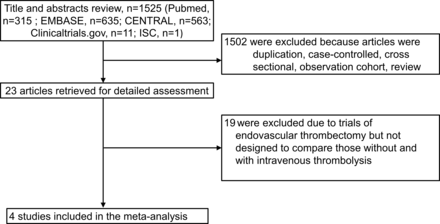
The formal literature search identified 22 full articles for detailed assessment, of which 19 were excluded for not being designed to compare EVT alone versus combined IVT plus EVT (figure 1). Our final analysis included four RCTs, enrolling 1633 individuals, with 817 (50%) participants randomly assigned to the EVT alone and 816 (50%) to IVT before EVT.12 17–19 Three trials have published formal articles12 17 18 and one trial was presented at the International Stroke Conference on March 18, 2021, pending formal publication.19 Inclusion criteria and baseline characteristics of these trials are shown in table 1. Three trials were conducted in Asia, two in China,12, 18 and one in Japan,17 while one trial was conducted in European countries (Netherlands, France, and Belgium).19 All enrolled patients with AIS-LVO in the anterior circulation presenting directly to the thrombectomy-capable hospital. Across all trials, mean age ranged from 69 to 76 years, 58% of patients were male, and median National Institutes of Health Stroke Scale (NIHSS) score at baseline ranged from 16 to 19. Average duration from stroke onset to arterial puncture was 5 to 15 min shorter in the EVT alone group compared with combined IVT plus EVT group across trials. Some baseline characteristics varied among included trials. In the DIRECT-MT trial,18 DEVT trial,12 and MR CLEAN-NO IV trial,19 83% to 96% of occlusions were located on the internal carotid artery (ICA) or M1 middle cerebral artery (MCA), a standard dose of alteplase (0.9 mg/kg) was used, and approximately 30–40 min occurred between IV alteplase start and arterial puncture. With their higher drug dose and longer interlude for drug action, these three trials more strongly probed the ability of IVT to improve outcome by quickly dissolving the target occlusion before EVT can be performed. Conversely, in the SKIP trial,17 only 60% of occlusions were located in the ICA or M1 MCA, a lower dose of altplase (0.6 mg/kg) was used, and 8 min occurred between IVT start and arterial puncture (with 21% of patients even receiving arterial puncture before IVT). Accordingly, the SKIP trial could be considered to have more closely explored the possibility that lower-dose, shorter-interlude IV alteplase can improve outcome by dissolving residual small thrombi in the distal vasculature after incomplete endovascular reperfusion. In three of the trials (DIRECT MT, SKIP, DEVT), the prespecified primary evaluation was a non-inferiority analysis; in one of the trials (MR CLEAN-NO IV), the prespecified primary evaluation was a superiority analysis and a non-inferiority analysis was a prespecified secondary evaluation.
Flow of study selection. ISC, International Stroke Conference.
Characteristics of included trials
Risk of bias was low for all trials except possible performance bias due to inadequate blinding of participants/personnel (online supplemental efigure 1).
Supplemental material
For the primary efficacy endpoint of functional independence (mRS 0–2) at 90 days, crude cumulative rates of functional independence were 46.0% with direct EVT versus 45.5% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference of functional independence was 1% (95% CI −4% to 5%) between EVT alone and IVT before EVT (figure 2). There was no heterogeneity among trials (I2=0%). The lower 95% CI bound of −4% fell within the non-inferiority margins of −15%, −10%, –6.5%, and −5%, but crossed the most stringent non-inferiority margin of −1.3%.
Functional independence. Forest plot comparing EVT alone versus IVT before EVT for functional independence (modified Rankin Scale 0–2). The lower 95% CI bound of −4% fell within the non-inferiority margins of −15%, −10%, –6.5%, and −5%, but crossed the most stringent non-inferiority margin of −1.3%. CI, confidence interval; EVT, endovascular thrombectomy; IVT, intravenous thrombolysis.
For the secondary clinical efficacy endpoint of freedom from disability (mRS 0–1) at 90 days, crude cumulative rates of freedom of disability were 25.6% with direct EVT versus 24.2% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference of freedom of disability was 1% (95% CI −3% to 5%) between EVT alone and IVT before EVT (figure 3). There was no heterogeneity among trials (I2=0%). The lower 95% CI bound of −3% fell within the non-inferiority margins of −15%, −10%, −6.5%, and −5%, but crossed the most stringent non-inferiority margin of −1.3%.
Freedom of disability. Forest plot comparing EVT alone versus IVT before EVT for freedom of disability (modified Rankin Scale 0–1). The lower 95% CI bound of −3% fell within the non-inferiority margins of −15%, −10%, –6.5%, and −5%, but crossed the most stringent non-inferiority margin of −1.3%. CI, confidence interval; EVT, endovascular thrombectomy; IVT, intravenous thrombolysis.
For the secondary technical efficacy endpoint of successful reperfusion at end of procedure, crude cumulative rates were 76.5% with direct EVT versus 81.0% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference for successful reperfusion was −4% (95% CI −8% to 0%), suggesting EVT alone was inferior to IVT before EVT (online supplemental efigure 2).
For the hemorrhagic transformation safety endpoints, crude cumulative rates of any intracranial hemorrhage were 27.8% with direct EVT versus 36.3% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference for any intracranial hemorrhage was 9% (95% CI 3% to 15%), favoring EVT alone over IVT before EVT (figure 4A). Crude cumulative rates of symptomatic intracranial hemorrhage were 4.9% with direct EVT versus 5.8% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference for symptomatic intracranial hemorrhage was 1% (95% CI −1% to 3%) between EVT alone and IVT before EVT (figure 4B).
Intracranial hemorrhage. Forest plot comparing EVT alone versus IVT before EVT for (A) any intracranial hemorrhage and (B) symptomatic intracranial hemorrhage. CI, confidence interval; EVT, endovascular thrombectomy; IVT, intravenous thrombolysis.
For the safety outcome of all-cause mortality by 90 days, crude cumulative rates were 17.4% with direct EVT group versus 16.4% with bridging IVT plus EVT. Pooled results from the random-effect model showed the risk difference for all-cause mortality was −1% (95% CI −4% to 3%) between direct EVT and bridging IVT before EVT (online supplemental efigure 3).
Discussion
The current meta-analysis comprising four RCTs with 1633 patients found strong indications that direct EVT alone is non-inferior to bridging IVT plus EVT for anterior circulation AIS-LVO. For both the primary clinical efficacy outcome of functional independence (mRS 0–2) at 90 days and the secondary clinical efficacy outcome of freedom from disability (mRS 0–1) at 90 days, point estimates favored direct EVT and criteria for statistically significant demonstration of non-inferiority of direct EVT were met for established non-inferiority margins of −15%, −10%, −6.5%, and −5%. However, the pooled results did not demonstrate statistically significant non-inferiority for the most stringent non-inferiority margin of −1.3%. Also, EVT alone compared with IVT before EVT had a lower chance of achieving end of procedure successful reperfusion, but had a lower risk of any intracranial hemorrhage. Symptomatic intracranial hemorrhage and all-cause mortality were not different between both groups.
Results of the comparison between EVT alone versus IVT before EVT based on prior meta-analyses of observational studies were inconsistent. One meta-analysis suggested IVT plus EVT compared with EVT alone had better functional outcomes, lower mortality, higher rate of successful recanalization, and similar odds of symptomatic intracranial hemorrhage.20 Another network meta-analysis suggested IVT before EVT compared with EVT alone for LVOs may not be associated with improved outcomes, including functional independence.21 Furthermore, patients treated with IVT before EVT have greater hospital encounter charges and final hospital bills than patients who undergo treatment with EVT only.22 Since biases are invariably more pronounced in observational studies than RCTs, it is more appropriate to use the results of trials, once available and congruent, to guide routine clinical practice. The pooled results from these trials suggest that the EVT alone strategy is broadly non-inferior to IVT before EVT and accordingly might be reasonable to consider for some patients who present directly to hospitals capable of performing EVT.
In the current meta-analysis, we assessed five different non-inferiority margins established in the literature, rather than a single margin. The three least stringent margins were selected as having been in acute stroke trials with non-inferiority designs. All had been chosen using the ‘fixed margin’ approach to identifying a non-inferiority margin. The fixed margin method does not aim to demonstrate definitive non-inferiority in the strongest sense of formally excluding the minimal clinically important difference. This approach is undertaken when a trial powered to demonstrate definitive non-inferiority is deemed infeasibly large. The goal of fixed margin trials is show that the newer treatment delivers at least a substantial fraction of the benefit of the standard treatment.15 23 24 With the fixed margin approach, it is more accurate to say a trial’s aim is to show that the new treatment is reasonably comparable to the standard treatment, rather than non-inferior and to consider the selected threshold a ‘reasonably comparable margin’ rather than a ‘non-inferiority margin’.15 The two more stringent margins were selected as having been identified in acute stroke expert survey studies as indicating the minimally clinically important difference for dichotomized mRS outcomes. The goal of such margins is to demonstrate definitive non-inferiority, namely that the new treatment has effects clinically indistinguishable from the standard treatment.
The approach of presenting a framework of five different, rather than a single, non-inferiority margin enables clinicians to determine individually if they feel that non-inferiority has been demonstrated by accumulated results. In the current meta-analysis, the lower bound of the 95% CI for mRS 0–2 at 90 days is −4%, indicating that the data do not exclude that, among 100 treated patients, four fewer would achieve functional independence if treated with direct EVT rather than bridging IVT plus EVT. As result, for clinicians who personally consider a treatment as not being inferior if it yields 15, 10, 6.5, or 5 fewer independent outcomes among 100 patients at 90 days, non-inferiority has been statistically demonstrated. However, for clinicians who personally consider a treatment as not being inferior if it yields 1.3 fewer independent outcomes among 100 patients at 90 days, non-inferiority has not yet been statistically demonstrated.
Interestingly, although point estimates for clinical outcomes favored direct EVT, the current meta-analysis demonstrated higher rates of successful reperfusion in patients receiving IVT before EVT compared with EVT alone. Several factors may contribute to this apparently paradoxical result. First, excellent reperfusion (≥90%, eTICI 2c-3) is a better predictor of good clinical outcome than is successful reperfusion (≥50%, eTICI 2b50-3).9 Rates of end of procedure excellent reperfusion have not yet been reported from all of the completed trials. Second, bridging IVT plus EVT may have been associated with longer times to achieve successful reperfusion, even though it eventually yields more frequent reperfusion. Time to first successful reperfusion has not yet been reported from all of the completed trials. Third, the higher rates of asymptomatic intracranial hemorrhage and tendency to more symptomatic intracranial hemorrhage among bridging IVT plus EVT patients may offset the benefits of higher reperfusion rates.
There are limitations to this study. First, some baseline characteristics varied among included trials, as presented in the Results section. In part because of these differences in trial design and conduct, we undertook a random rather than fixed effects meta-analysis. Also, there was no heterogeneity of treatment effect among trials. Second, the current trials provide information only regarding IV alteplase as the thrombolytic strategy. Use of new IV fibrinolytic agents like tenecteplase before EVT,25 as well as IV fibrinolytic agents combined with IV glycoprotein IIb/IIIa inhibitors or direct thrombin inhibitors,26 continues to be an important avenue for further therapeutic study. Third, these trials were conducted in Asia and European countries and generalizability of the current results to certain population, such as African Americans, is not known. Additional trials, including SWIFT-DIRECT (NCT03192332) and DIRECT-SAFE (NCT03494920), are underway to clarify whether these findings can be generalized to all patients. Fourth, as the trials reported patient subgroup analyses using different outcomes (mRS shift for DIRECT MT and MR CLEAN-NO IV, mRS 0–2 for DEVT and SKIP), study-level meta-analysis for heterogeneity of treatment effect by different patient factors could not be undertaken. Individual participant data meta-analyses of all available trials would be particularly useful. Finally, reflecting entry criteria of all the analyzed RCTs, results from the current meta-analysis apply only to patients treated who arrive directly at a thrombectomy-capable hospital so that they transition from door to IVT to EVT fairly rapidly (‘mothership’ patients). These data cannot be applied to individuals arriving first at a non-thrombectomy center where IVT may be given and then transferred to a thrombectomy center for EVT, resulting in an extended interval between IVT and EVT (‘drip-and-ship’ patients).
In conclusion, meta-analysis of accumulated clinical trial data suggests that direct EVT has now been demonstrated to be statistically non-inferior to bridging IVT and EVT for several, but not the most stringent, non-inferiority margins. The available data are not definitive due to lack of published data in certain populations, such as African Americans, and lack of statistical significance for the most stringent non-inferiority margin. Still, the current RCT evidence suggests that it may sometimes be reasonable to skip IVT, and instead proceed with a strategy of rapid direct EVT in AIS-LVO patients who present directly to hospitals capable of performing EVT.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors C-HL: acquisition of data, analysis and interpretation of data, wrote the first draft. JS: study supervision, interpretation of data, critical revision of manuscript for intellectual content. BO: critical revision of manuscript for intellectual content. W-YH: analysis and interpretation of data. ML: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. Transparency declaration: ML, the corresponding author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding This work was supported by Ministry of Science and Technology, Taiwan, grant number: MOST 108-2314-B-182-017, 109-2314-B-182-033 and Chang Gung Memorial Hospital, Taiwan, grant number: CMRPG6H0441, CORPG6G0191, CORPG6G0192, CORPG6G0193. The sponsors played no role in the study design, data collection and analysis, or decision to submit the article for publication.
Competing interests Dr Saver reported being an employee of the University of California, which has patent rights in retrieval devices for stroke. The University of California received payments on the basis of clinical trial contracts for the number of participants enrolled in multicenter clinical trials sponsored by Medtronic, Stryker, Cerenovus, BrainsGate, NONO Inc., and Boehringer Ingelheim (prevention only). The University of California receives grant support from the National Institutes of Health (NIH) for Dr Saver’s service in leadership roles in the National Institute of Neurological Disorders and Stroke StrokeNet national clinical trial network and from Diffusion Pharma for Dr Saver’s leadership role in the PHAST-TSC multicenter trial. Dr Saver reported serving as an unpaid consultant to Genentech advising on the design and conduct of the PRISMS trial; neither the University of California nor Dr Saver received any payments for this voluntary service. Dr Saver paid for his own travel. Dr Saver reported receiving contracted hourly payments and travel reimbursement for services as a scientific consultant advising on rigorous trial design and conduct to Medtronic, Stryker, Cerenovus, BrainsGate, Boehringer Ingelheim (prevention only), NONO Inc., BrainQ, and Abbott; contracted stock options for services as a scientific consultant advising on rigorous trial design and conduct to Rapid Medical; and personal fees from Johnson & Johnson and Novo Nordisk.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.