Article Text
Abstract
Background WEB treatment is an endovascular approach for wide-neck bifurcation aneurysms that has demonstrated high safety and good efficacy in mid-term follow-up. While evaluating safety in the long term is important to determine if delayed adverse events occur affecting late morbidity and mortality, the most important point to evaluate is the long-term stability of aneurysm occlusion. The current analysis reports the 3-year clinical and anatomical results of WEB treatment in the combined population of two European trials (WEBCAST (WEB Clinical Assessment of Intrasaccular Aneurysm Therapy) and WEBCAST-2).
Methods Aneurysm occlusion was evaluated using a 3-grade scale: complete occlusion, neck remnant, and aneurysm remnant.
Results The safety population comprised 79 patients. The efficacy population comprised 61 aneurysms. Aneurysm locations were middle cerebral artery in 32/61 aneurysms (52.5%), anterior communicating artery in 13/61 (21.3%), basilar artery in 9/61 (14.8%), and internal carotid artery terminus in 7/61 (11.5%). No adverse events related to the device or procedure occurred between 2 and 3 years. At 3 years, complete occlusion was observed in 31/61 (50.8%) aneurysms, neck remnant in 20/61 (32.8%), and aneurysm remnant in 10/61 (16.4%). Between 1 year and 3 years, aneurysm occlusion was improved or stable in 53/61 (86.9%) aneurysms and worsened in 8/61 (13.1%). Worsening was mostly from complete occlusion to neck remnant in 6/61 (9.8%) aneurysms. The retreatment rate at 3 years was 11.4%.
Conclusions This analysis confirms the high safety profile of WEB. Moreover, evidence demonstrates the great stability of aneurysm occlusion with adequate occlusion (complete occlusion or neck remnant) in 83.6% of aneurysms.
Clinical trial registration URL: http://www.clinicaltrials.gov. WEBCAST and WEBCAST-2: Unique identifier: NCT01778322.
- aneurysm
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Intracranial aneurysm (IA) treatment aims to prevent rebleeding in cases of ruptured IA, and bleeding in cases of unruptured IA. As shown in the CARAT study, risk of rebleeding of treated ruptured aneurysms is related to the quality of aneurysm occlusion, with a rebleeding rate of 1.1% for total occlusion, 2.9% for small residual neck, 5.9% for neck remnant, and 17.6% for aneurysm remnant.1 CARAT also shows that risk of rerupture tends to be greater after aneurysm coiling compared with aneurysm clipping. The quality of any aneurysm treatment is determined by evaluating short- and long-term safety and efficacy. Safety depends on adverse events that typically happen during the procedure, but can also be delayed.2 3 Short- and long-term efficacy must also be evaluated given that aneurysm coiling is associated with a relatively high risk of aneurysm reopening (approximately 20%) that can lead to aneurysm bleeding/rebleeding.4 Thus, evaluating new aneurysm treatment should include a precise analysis of procedural and post-operative complications, morbidity, and mortality, while evaluating efficacy over the short (6–12 months) and long term.
Although surgery and clipping was the first efficacious aneurysm treatment, after ISAT (International Subarachnoid Aneurysm Trial), these treatments were replaced by endovascular treatment, which initially comprised coiling (with or without balloon assistance).5 Given the complexity of treating some intracranial aneurysms with coiling or balloon-assisted coiling (BAC), alternative techniques such as flow diversion and flow disruption soon developed.6 7 Flow disruption with WEB is an innovative treatment that has been evaluated in several single-center and multi-center prospective and retrospective studies and has shown high safety and efficacy in the short term.8–11 Indeed, since introduction of the device in Europe in 2010, several Good Clinical Practice (GCP) studies have been conducted, including two European trials (WEBCAST (WEB Clinical Assessment of Intrasaccular Aneurysm Therapy) and WEBCAST-2), one trial in the United States (WEB-IT [Intra-saccular Therapy]), and one French trial (French Observatory), confirming with rigorous methodology the short- and mid-term safety and efficacy of this technique.12–17 Furthermore, 2 years of follow-up in the cumulated population of the three European studies (WEBCAST, WEBCAST-2, and French Observatory) has confirmed the high safety and efficacy of this treatment.18
Given that two of the three European GCP studies (WEBCAST and WEBCAST-2) required follow-up at 3 and 5 years, the present study analyzes the clinical and anatomical results at 3 years of WEB aneurysm treatment in the combined population of WEBCAST and WEBCAST-2 trials.
Materials and methods
WEBCAST and WEBCAST-2 are single-arm, prospective, consecutive, multi-center European trials dedicated to evaluating WEB treatment for wide neck bifurcation aneurysms.
Both trials received national regulatory authorization according to each country’s regulations. In France, the study was approved by CCTIRS (Consultative Committee of Information Processing in Healthcare Research Program), the Reims Institutional Review Board, and CNIL (National Commission for Data Processing and Freedom). Written informed consent was obtained from all patients. In Germany, the study was approved by local ethics committees of participating centers.
Web devices
The WEB is a self-expanding, retrievable, electrothermally detachable, nitinol braided device, which is placed within the aneurysm sac. During the study time frame, the device existed in several iterations: initially WEB dual layer (WEB DL), followed by a single layer in two shapes (barrel: WEB SL, and spherical: WEB SLS), followed by a version that enhanced visualization (WEB SL EV, and WEB SLS EV) by incorporating composite wire strands made from nitinol and platinum.
In tandem with device developments, the microcatheters used to deliver these devices also evolved. Initially, the WEB DL was delivered according to device size using Headway 27 (Microvention, Tustin, California) or DAC 038 (Stryker, Fremont, California). Later, specific microcatheters were developed for WEB treatment including the VIA 27 and VIA 33 (Microvention/Sequent Medical, Aliso Viejo, California). Recently, the VIA 21 and 17 microcatheters (Microvention/Sequent Medical, Aliso Viejo, California) were introduced for WEB sizes between 3–7 mm.
Trial design and procedural modalities
Trial design and procedural modalities have been described in previous publications, and both studies were conducted following GCP guidelines.12–15 17 18 Inclusion criteria for the two studies were: ruptured (Hunt & Hess I, II, or III) and unruptured aneurysms located in the basilar artery (BA) apex, middle cerebral artery (MCA) bifurcation, internal carotid artery terminus (ICAt), or anterior communicating artery complex (Acom). Trial participants at each center received endovascular treatment (EVT) as first-line treatment as indicated by a local multidisciplinary team that included neurosurgeons and neuroradiologists. Selection of aneurysms treated with the WEB device was performed autonomously in each center by interventional neuroradiologists based on aneurysm characteristics. Procedural modalities were described in previous publications.17 18
Data collection
Each center completed a patient file with the following data:
Demographics: age and gender;
Aneurysm: rupture status, location, size, and neck size;
Procedure: date, type of device used (DL or SL/SLS), and occurrence of complications during or after procedure.
Preoperative Hunt and Hess grade was collected with ruptured aneurysms. Modified Rankin Scale score (mRS) was gathered before treatment for unruptured aneurysms. In addition to clinical follow-up at 30 days (±7 days), clinical and imaging follow-up was mandatory at 1, 3, and 5 years.
Data analysis
Clinical data, including all adverse events, were independently monitored and analyzed by a single medical monitor (AM). An expert interventional neuroradiologist (JB) independently evaluated aneurysm location on initial angiogram. Aneurysm occlusion was evaluated using a 3-grade scale: complete occlusion, neck remnant, and aneurysm remnant. This evaluation was performed on post-operative digital subtraction angiography (DSA), and on 1- and 3-year vascular imaging (DSA, MRA, or CTA), which was selected autonomously by the centers. No specific examination protocol was recommended for these different techniques. Based on previous work, opacification of the proximal recess of the WEB device was considered as complete occlusion.11 Evolution of aneurysm occlusion between 1 and 3 years was also evaluated using a 3-grade scale: worse, stable, and improved. Worsening and improvement were defined as a grade change in the 3-grade occlusion scale.
Per protocol, patients who were retreated after the index procedure and before the follow-up object of the analysis were not included in safety and efficacy analyses as the goal of the study was to evaluate the performance of the WEB itself.
Missing data
For the patients who had no follow-up vascular imaging at 3 years, the following information was collected:
Reason for not having this examination,
Date of the last follow-up vascular imaging before 3 years (1 or 2 years),
Status of the aneurysm occlusion at the last follow-up vascular imaging before 3 years.
Statistical analysis
Continuous variables were described as mean±SD. Categorical data were described numerically as a categorical total and as a percentage of the analyzed population. Binomial data were described as a ratio of the true value and the analyzed population (x/n). Confidence intervals for binomial data were calculated by the Clopper–Pearson method, and P-values were calculated by the Fisher Exact Test. Analyses were conducted using SPSS statistical software (SPSS, Chicago, Illinois.).
Results
Patient and aneurysm population
The combined population of the two trials included 106 patients aged 27 to 77 years (mean: 55.0±10.4 years). The 3-year follow-up visits were performed between 32 and 46 months (mean: 36.8±2.5 months) after initial procedure. According to the study flow chart (figure 1), the study population for safety comprised 79/106 patients (74.5%), including 53 females (67.1%), with age range of 30–77 years (mean: 54.4±10.2 years). If we take into account (figure 1), patients for whom WEB safety cannot be evaluated (no WEB implanted [five] and consent withdrawn [four]) and those who were retreated (nine), the percentage of patients with 3-year clinical follow-up is 89.8% (79/88 patients).
Population flow chart for two studies for safety and efficacy at 3 years. * This patient had no vascular follow-up imaging at 1 year, but had it at 3 years. "lost to follow-up" means that the centers were unable to contact the patients using different techniques (phone call, email, letter, etc) so the patient exited the study. "missed visits" means that the patient did not attend the 3-year follow-up, but attended a later visit.
The study population for efficacy (figure 1) comprised 61/106 (57.5%) aneurysms. If we take into account (figure 1), patients for whom WEB efficacy cannot be evaluated (no WEB implanted [five], consent withdrawn [four], death [five]) and those who were retreated (nine), the percentage of patients with 3 years' anatomical follow-up is 73.5% (61/83 patients). Aneurysm status was ruptured in 2/61 (3.3%) and unruptured in 59/61 (96.7%). Aneurysm locations per core laboratory analysis were MCA in 32/61 aneurysms (52.5%), Acom in 13/61 (21.3%), BA in 9/61 (14.8%), and ICAt in 7/61 (11.5%). Aneurysm size ranged from 3.6 to 12.0 mm (mean: 6.9±1.7 mm). The neck was wide (≥4 mm) in 52/61 (85.2%) aneurysms.
Morbidity and mortality at 3 years
There were no delayed complications reported during the 2–3 year period following the initial procedure, and no hemorrhagic or thromboembolic events were observed. At 3 years, 1/79 (1.3%) patient had morbidity unrelated to the initial procedure (alcoholic neuropathy). Total number of deaths at 3 years was 5/79 (6.3%), including one procedure-related (retroperitoneal hematoma) and four unrelated to aneurysm or procedure (three patients with cancer, one with pneumonia).
Anatomical results at 3 years
Vascular imaging techniques included DSA in 16/61 aneurysms (26.2%), magnetic resonance angiography (MRA) in 41/61 (67.2%) aneurysms, and CT angiography (CTA) in 4/61 (6.6%) aneurysms. At 3 years, complete occlusion was observed in 31/61 (50.8%) aneurysms (figure 2), neck remnant in 20/61 (32.8%), and aneurysm remnant in 10/61 (16.4%). Adequate occlusion (complete occlusion or neck remnant) was observed in 51/61 (83.6%) aneurysms. Of note, no neck or aneurysm remnant was associated with bleeding/rebleeding during the follow-up period.
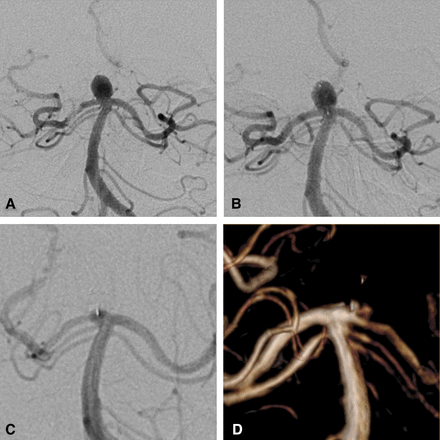
Unruptured basilar tip aneurysm. a: DSA (frontal view) before web treatment shows the aneurysm. b: post-operative DSA (frontal view) shows residual flow in the aneurysm and the device. c: 1-year DSA shows complete aneurysm occlusion. d: 3-year MRA shows stable complete aneurysm occlusion.
Compared with 1-year aneurysm occlusion, occlusion at 3 years was improved in 6/61 (9.8%) aneurysms, stable in 47/61 (77.0%), and worsened in 8/61 (13.1%). Worsening of aneurysm occlusion evolved from complete occlusion to neck remnant in 6/61 (9.8%) aneurysms, complete occlusion to aneurysm remnant in 1/61 (1.6%) aneurysms, and neck remnant to aneurysm remnant in one (1.6%) aneurysm.
Missing data
At 3 years, 17 patients had no vascular follow-up imaging available (figure 1) for the following reasons: imaging not done per institution’s standard of care in 12 patients, unavailability of the patient in two patients, renal problem in one patient, follow-up imaging performed in another institution than the institution where the index procedure was performed in one patient, and decision of the investigator in one patient.
Among these 17 patients, the last follow-up vascular imaging available was performed at 1 year in seven patients and 2 years in 10 patients.
In the seven patients with 1-year follow-up, the status of aneurysm occlusion according to the core laboratory was complete occlusion in six patients and aneurysm remnant in one patient. In the 10 patients with 2-year follow-up, the status of aneurysm occlusion according to the core laboratory was complete occlusion in eight patients, neck remnant in one patient, and aneurysm remnant in one patient. In the 17 patients, the status of aneurysm occlusion at 1 or 2 years was complete occlusion in 14/17 patients (82.4%), neck remnant in 1/17 patient (5.9%), and aneurysm remnant in 2/17 patients (11.8%).
Anatomical results at 3 years in aneurysms treated with WEB DL and WEB SL/SLS
No significant differences were observed in anatomical results for aneurysms treated with WEB DL and WEB SL/SLS (table 1).
Aneurysm occlusion at 3 years in aneurysms treated with WEB DL and WEB SL/SLS
Anatomical results at 3 years in different aneurysms locations
No significant differences were observed in anatomical results based on aneurysm location (table 2).
Aneurysm occlusion at 3 years according to aneurysm location
Retreatment
Retreatment rate was evaluated in 88 aneurysms. Excluded from this analysis were five aneurysms untreated with WEB, four aneurysms of four patients who withdrew consent, and nine aneurysms in patients lost to follow-up or without follow-up performed at 3 years. Four (4.5%) aneurysms were retreated (or had a retreatment attempt) between initial procedure and 1 year, four (4.5%) between 1 year and 2 years, and two (2.3%) between 2 years and 3 years leading to a retreatment rate at 3 years of 10/88 (11.4%). Retreatment modalities were stent (with or without coils) in six aneurysms (figure 3), flow diverter in two aneurysms, and clipping in two aneurysms (clipping failed in one case).
Unruptured middle cerebral artery aneurysm. a: DSA (frontal view) shows the aneurysm. b: 1-year DSA (unsubstracted, non injected) shows web retraction. c:1-year DSA shows an aneurysm remnant. d: retreatment by stent-assisted coiling. e: final control DSA after retreatment shows complete aneurysm occlusion.
Discussion
The combined population of two European GCP WEB trials (WEBCAST, WEBCAST-2) represents the largest prospective series to date of patients treated with the WEB device including long-term follow-up. Obtaining long-term follow-up in a large, multi-center patient cohort is usually difficult: however, these studies collected 3 years' clinical follow-up (safety) in 74.5% of patients and 3 years' anatomical follow-up (efficacy) in 57.5%. No adverse events occurred between 2 and 3 years, and the global morbidity and mortality at 3 years were 1.3% and 6.3%, respectively. Of note, morbidity was not related to initial procedure and only one death (1.3%) was related to the initial procedure. At 3 years, anatomical results were complete aneurysm occlusion in 50.8%, neck remnant in 32.8%, and aneurysm remnant in 16.4% with adequate occlusion (complete occlusion or neck remnant) in 83.6% and retreatment in 11.4%.
The current analysis in two GCP studies shows low morbidity at 3 years (1.3%), which is similar to what was reported in three GCP populations at 1 month (3.0%), 1 year (1.3%), and 2 years (1.4%).17 18 As analyzed in previous papers, initial morbidity was typically related to subarachnoid hemorrhage severity in cases of ruptured aneurysm and to intra-operative complications, singularly thromboembolic events.17 As some patients progressively improved between 1 month and 1 year (and beyond), morbidity rates decreased and stabilized over time to a very low level (around 1%). Mortality rates in the three GCP populations was zero at 1 month, similar to reports from the US trial (WEB-IT).16 17 Overall, mortality progressively increased (3.3% at 1 year, 3.6% at 2 years) and, at 3 years, mortality in the two GCP populations was 6.3%: however, most deaths resulted from diseases (cancer and infection) unrelated to the procedure.17 18 Of note, no delayed adverse events between 1 year and 3 years were reported, in contrast to what is observed with flow diversion.18
Long-term follow-up also provides valuable data on stability of aneurysm occlusion. However, comparing techniques is not easy. In short, relatively little is known regarding aneurysm occlusion stability after clipping, given that most series do not perform imaging follow-up.19 In a recent series of patients with MCA aneurysms treated by clipping, the authors mention that “patients only underwent delayed angiographic examination if they had subtotal occlusion of their aneurysm on intraoperative DSA”.20 After coiling, aneurysm occlusion is not stable (recanalization) in a percentage of patients, which is variable from one series to another (14.8% to 33.6%).21 A large meta-analysis showed that aneurysm reopening occurred in 20.8% of coiled aneurysms, requiring retreatment in approximately 10% of aneurysms.4 Stent-assisted coiling (SAC) is often associated with better anatomical results, but has not been confirmed by a randomized trial. Even though flow diversion is associated with a relatively high percentage of complications, it is also the endovascular technique aligned with the highest rate of complete aneurysm occlusion at long-term follow-up.22 23 In the Pipeline for Uncoilable or Failed Aneurysms (PUFS) trial, the rate of complete aneurysm occlusion at 3 years was 93.4%, with a rate of retreatment of 6.6%.23 What is more, no recanalization was observed at 3 years.
As flow diversion and flow disruption do not share similar indications (the use of flow diverters in bifurcation aneurysms remains a matter of debate), efficacy of WEB aneurysm treatment is usually compared with coiling (including BAC and SAC). Nevertheless, comparing aneurysm occlusion in patients treated with WEB and other endovascular techniques based on published data is not easy since most series dedicated to aneurysm coiling (including BAC and SAC) combine all types of aneurysms – small, large, and giant; narrow and wide necked; as well as bifurcation and sidewall aneurysms. For example, the Matrix and Platinum Science (MAPS) trial conducted an analysis in the subgroup with wide-neck aneurysms showing that complete aneurysm occlusion and adequate occlusion at 1 year were obtained in 18.6% and 45.7%, respectively, after aneurysm coiling, and 27.1% and 57.6%, respectively, after SAC, which is worse when compared with WEB results at 3 years in the present series.24 Similarly, the BRANCH trial (wide-neck bifurcation aneurysms of the middle cerebral artery and the basilar apex treated by endovascular techniques) showed rates of complete aneurysm occlusion and adequate occlusion in patients treated with coiling (including BAC and SAC) for unruptured wide-neck bifurcation aneurysms of 30.6% and 63.0%, respectively, at a mean follow-up of 48.8 weeks.25 With complete occlusion in 50.8% and adequate occlusion in 83.6% at 3-year follow-up, it seems beneficial to use the WEB device in the wide-neck bifurcation aneurysm subgroup, but these treatments need direct comparison. Importantly, anatomical results are similar in patients with dual-layer and single-layer devices (WEB DL vs WEB SL/SLS), indicating that this important technical evolution that has made treatment easier has not had negative impacts on device efficacy.
Retreatment rate at 3 years from initial procedure was 11.4% of aneurysms, which is comparable to data reported at 1 year in the MAPS trial for aneurysms treated with coils (13.7%) or stent-assisted coiling (14.1%). Endovascular techniques used for retreatment were stenting (with or without associated coils) or flow diversion, but clipping was also an option.26
Limitations
This study has several limitations. First, it is not a randomized study, which makes comparing results with other techniques difficult: however, the study shows that safety and efficacy (aneurysm occlusion) is not altered with time. Second, the percentage of patients with anatomical follow-up was not very high (57.5%) in comparison with clinical follow-up (74.5%). Nonetheless, this independent analysis is the first study to evaluate aneurysm occlusion 3 years after WEB treatment in a prospective, multicenter population with a reasonable number of patients. Moreover, when taking into account patients for whom follow-up was not feasible (no WEB implanted, consent withdrawn, and death) or patients who were retreated, the effective percentage of clinical and anatomical follow-up at 3 years is 89.8% and 73.5%, respectively. As there is some concern regarding the 17 patients who have no follow-up vascular imaging available at 3 years, the last anatomical data available for them (at 1 or 2 years) were retrieved and are complete occlusion in 14/17 patients (82.4%), neck remnant in 1/17 patient (5.9%), and aneurysm remnant in 2/17 patients (11.8%). According to the proportion of every occlusion status, these missing data are probably not able to significantly modify the global results of the manuscript, even if there was some worsening of aneurysm occlusion between 1 or 2 years and 3 years. Third, imaging modalities used for the 3-year follow-up were heterogeneous including DSA, MRA, and CTA. As DSA is an invasive technique associated with some rare complications, it cannot be proposed for the long-term follow-up of aneurysms treated with WEB, except if other techniques show worsening of aneurysm occlusion. On the other hand, MRA and CTA were previously evaluated for the follow-up of patients treated with WEB, with acceptable results.27 28
Conclusion
Three-year follow-up of patients treated with WEB in two prospective, multi-center WEB trials (WEBCAST and WEBCAST-2) confirms the high safety of the device. The most important finding is the high level of stability of aneurysm occlusion after 3 years with adequate occlusion in 83.6% and a global retreatment rate of 11.4%.
References
Footnotes
Contributors All authors have: provided a substantial contribution to the conception and design of the studies and/or the acquisition and/or the analysis of the data and/or the interpretation of the data; drafted the work or revised it for significant intellectual content; approved the final version of the manuscript; and agreed to be accountable for all aspects of the work, including its accuracy and integrity.
Funding WEBCAST and WEBCAST-2 were funded by Sequent.
Competing interests LP consults for Balt, MicroVention, Neuravi, and Penumbra. ISI consults for Codman, Medtronic, Sequent, and Stryker. XB consults for MicroVention and Stryker. LS consults for Stryker, MicroVention, Medtronic, and Balt. JF has received fees as consultant or lecturer from Acandis, Bayer, Boehringer-Ingelheim, Codman, Covidien, MicroVention, Penumbra, Philips, Sequent, Siemens, and Stryker; his institution received funding from MicroVention, Medtronic, BMBF, BMWi, DFG, and the EU. VC consults for MicroVention and Balt, and receives educational grants from Medtronic and Stryker. JK consults for MicroVention/Sequent. WW consults for MicroVention, Phenox, and Medtronic. TL consults for Medtronic, Mentice, Microvention, and Route 92. JM consults for Medtronic, MicroVention, Stryker, and Balt. AM consults for MicroVention/Sequent and Cerus Endovascular. JB consults for, and is shareholder of, Oxford Endovascular Ltd; his institution received funding from MicroVention.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.