Article Text
Abstract
Background Vessel perforation during stent retriever thrombectomy is a rare complication; typically only single instances have been reported.
Objective To report on a series of patients whose stent retriever thrombectomy was complicated by intraprocedural vessel perforation and discuss its potential mechanisms, rescue treatment strategies, and clinical significance.
Methods Cases with intraprocedural vessel perforation, where a stent retriever was used either as a primary treatment approach or as a part of a direct aspiration first pass technique (ADAPT), were included in the final analysis. Clinical data, procedural details, radiographic and clinical outcomes were collected from nine participating centers.
Results Intraprocedural vessel perforation during stent retriever thrombectomy occurred in 16 (1.0%) of 1599 cases. 63% of intraprocedural perforations occurred at distal locations. Endovascular rescue techniques (most commonly, intracranial balloon occlusion for tamponade) were attempted in 50% of cases. Procedure was aborted without any rescue attempts in 44% of cases. Mortality during hospitalization and at 3 months was 56% and 63%, respectively. 25% of patients achieved good functional outcome at 3 months after the procedure.
Conclusions Intraprocedural perforations during stent retriever thrombectomy were rare, but when they occurred were associated with high mortality. Perforations most commonly occurred at distal occlusion sites and were often characterized by difficulty traversing the occlusion with a microcatheter or microwire, or while withdrawing the stent retriever. Nevertheless, 25% of patients had a favorable functional outcome, suggesting that in some patients with this complication good neurological recovery is achievable.
- Stroke
- Subarachnoid
- Thrombectomy
Statistics from Altmetric.com
Introduction
Stent retriever thrombectomy has proved to be safe and effective for the treatment of acute stroke from large vessel occlusion based on results of the recent randomized trials of endovascular stroke therapy.1–5 Analysis of procedural complications associated with stent retriever thrombectomy showed its overall rate was lower than with early generation mechanical thrombectomy devices, and most commonly included intracranial hemorrhage (ICH), vasospasm, emboli to new territories, arterial dissection, and serious groin complications.6–8 The proposed mechanisms of ICH include direct endoluminal trauma and shear forces on the perforator vessels causing subarachnoid hemorrhage (SAH), and reperfusion injury resulting in parenchymal hematoma formation.9–11
Vessel perforation during stent retriever thrombectomy is a rare complication; with only single instances being reported in studies and case series.8 ,12 A comprehensive review of predisposing factors and potential mechanisms for onset of the complication, clinical outcomes, and rescue treatments is lacking. In this multicenter retrospective review, we report on a series of patients whose stent retriever thrombectomy was complicated by intraprocedural vessel perforation.
Methods
The study was approved by the local institutional review board at each of the participating centers for retrospective data collection and review. Each participating center retrospectively collected data on consecutive patients with acute ischemic stroke who had undergone IA revascularization using a stent retriever device between March 2012 and March 2016. All participating centers included only operators with neurointerventional fellowship training from three background specialties (neurosurgery, neurology, and/or neuroradiology). Centers with no stent retriever perforations during the study period were encouraged to participate and submit the total number of stent retriever thrombectomy cases to enable accurate calculation of the incidence of this complication in ‘real-world’ clinical practice. Cases with intraprocedural vessel perforation in which a stent retriever was used either as a primary treatment approach or as a part of a direct aspiration first pass technique (ADAPT) were included in the final analysis. Cases treated without the use of a stent retriever, such as those treated with primary aspiration thrombectomy alone or pharmacological thrombolysis, were excluded from the analysis. All participating centers used CT or magnetic resonance angiography for confirmation of large vessel occlusion as a part of screening for endovascular treatment.
The following data were collected: age, gender, cerebrovascular risk factors, admission National Institutes of Health Stroke Scale (NIHSS) score, time of symptom onset, and IV tissue plasminogen activator administration. Procedural technical details included location and characteristics of arterial occlusion, type of stent retriever, number of passes, final recanalization score, procedure duration, use of adjunct devices and pharmacologic agents, and description of rescue therapy and neurosurgical interventions, when applicable. Recanalization was defined according to a Thrombolysis in Cerebral Infarction (TICI) score. Functional neurologic outcomes were quantified using the modified Rankin scale (mRS) at 90 days. Revascularization and functional outcome data were reviewed by local investigators at each participating site and were not adjudicated by a central core laboratory. The Pearson correlation coefficient was used to study the relationship between stent retriever thrombectomy volumes at each center and the number of vessel perforation cases.
Using the PubMed database and search terms ‘perforation’, ‘complication’, ‘stent retriever’, ‘thrombectomy’, and ‘stroke’, we identified studies published between 1 January 2000 and 30 August 2016 that specifically reported the incidence of vessel perforation with mechanical thrombectomy.
Results
A total of 1599 patients from nine stroke centers were treated with IA thrombectomy using stent retrievers between March 2012 and March 2016. Two centers reported no incidence of vessel perforation and seven centers reported an average of two perforations per center. Intraprocedural vessel perforation during stent retriever thrombectomy occurred in 1.0% (16/1599) of patients. There was a weak positive relationship between the overall volume of stent retriever thrombectomy cases at each center and the number of cases with vessel perforation (R=0.088). Demographic data, distribution of vascular risk factors, stroke severity, and location of occlusion are summarized in table 1.
Baseline clinical characteristics, (n=16)
Technical details of each of the 16 thrombectomy cases, rescue approaches and outcomes are described in table 2. Ten of 16 (63%) intraprocedural perforations occurred at distal locations: middle cerebral artery (MCA) M2/3 (n=8), anterior cerebral artery A2 (n=1), and posterior cerebral artery P2/3 (n=1). The median number of stent retriever passes was 1 (IQR 1–2) before perforation was recognized. Once perforation occurred, procedure was aborted without any additional endovascular rescue maneuvers or additional thrombectomy attempts in 7 (44%) cases. In eight cases, endovascular rescue was pursued, including inflation of a balloon intracranially for tamponade (n=7) or flow arrest with a balloon guide catheter (n=1). In one case (patient 3), contrast extravasation was self-resolving and further thrombectomy attempts were continued. Six (38%) of patients had TICI 0, six (38%) had TICI 2a, and four (25%) had TICI 2b/3 reperfusion at the end of the procedure.
Summary of technical details of thrombectomy, rescue approaches and outcomes
Eight (50%) patients showed worsening at the neurologic examination within the first 24 hours after thrombectomy. Of those, six patients died in hospital, one patient had a poor outcome and one patient had a good outcome at 3 months. During hospitalization, mortality was 56% (9/16 patients). The overall mortality rate at 3 months was 63% (10/16 patients). Four (25%) patients achieved good functional outcome at 3 months. Below, we present two illustrative cases of patients with strokes whose thrombectomy was complicated by vessel perforation, and a description of the rescue approaches used.
Illustrative cases
Middle cerebral artery M1 occlusion
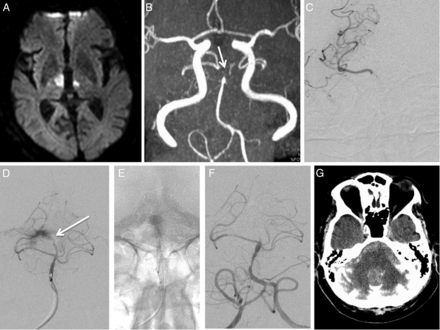
In this case of acute stroke from a right MCA M1 occlusion, the patient with a NIHSS score of 16, was first treated with IV thrombolysis and subsequently taken to the angiography suite (figure 1). Catheter angiography demonstrated persistent occlusion of the right MCA M1 segment. After two thrombectomy passes with the 4×20 mm device (Medtronic), persistent distal MCA occlusion was seen. Perforation at the M2/3 segment probably occurred owing to a loss of feedback from the microcatheter with the proximal end of the microcatheter forming a loop within the internal carotid artery. Contrast extravasation was self-limiting because the persistent clot provided tamponade of the ruptured segment. Thrombectomy was continued, and after two additional passes of the stent retriever, TICI 2b was achieved. A small amount of SAH was seen on postprocedural head CT without any signs of herniation or hydrocephalus. The patient was functionally independent (mRS=0) at 3 months.
(A) DSA shows occlusion of the M1 segment of the right middle cerebral artery (arrow). (B) A microcatheter is advanced into the M2/3 branch (arrow indicates the tip of the microcatheter), where active contrast extravasation pointing to the location of vessel perforation can be seen. Note that the microcatheter has formed a loop within the cervical internal carotid artery (arrowhead), which might have contributed to the loss of feedback while advancing the microcatheter, thus resulting in perforation. (C) After withdrawal of the microcatheter, contrast extravasation has stopped, indicating spontaneous cessation of bleeding. After two additional passes of the stent retriever device, Thrombolysis in Cerebral Infarction (TICI) 2b reperfusion is achieved. (D) After the procedure non-contrast CT shows a small amount of subarachnoid hemorrhage on the right side.
Top of the basilar artery occlusion
The patient presented with a NIHSS score of 25 as a wake-up stroke, with imaging suggesting a top of the basilar artery occlusion, based on bilateral distribution of thalamic strokes and lack of flow within the distal basilar artery (figure 2A, B). The patient was intubated en route for airway protection. While traversing the occluded right P1/2 posterior cerebral artery segment with a microcatheter, resistance was encountered. The microcatheter herniated back into the basilar artery while deploying the Solitaire stent retriever, and both were withdrawn. Contrast extravasation was observed on a subsequent injection (figure 2C, D). This was controlled by delivery and inflation of a HyperGlide balloon (Medtronic) inside the basilar artery (figure 2E, F). An external ventricular drain was placed and IV mannitol was administered. CT of the head showed a large amount of contrast and blood products (figure 2G). The patient remained intubated, never regained consciousness and subsequently died.
(A) MRI diffusion-weighted sequence shows a hyperintense signal in the thalamus bilaterally consistent with acute ischemic stroke. (B) Lack of flow at the top of the basilar artery is seen on MR angiography (arrow). (C) DSA, microcatheter is advanced into the right posterior cerebral artery, P1/2 segment. (D) While attempting to deliver and deploy the Solitaire stent retriever, the microcatheter herniated into the basilar artery. Angiography shows active contrast extravasation from perforation at the P1/2 junction. (E) A Hyperglide balloon was inflated for 5 min inside the basilar artery for hemostasis. Radiopaque markers of the balloon indicating its position within the basilar artery before inflation are shown. (F) DSA, follow-up injection shows no further contrast extravasation. (G) A large amount of contrast intermixed with blood products within the posterior fossa is seen on postprocedural head CT.
Discussion
Vessel perforation is a rare complication of stent retriever thrombectomy and its mechanisms, risk factors, outcomes, and rescue approaches have not been well described. Ten (1.6%) perforations occurred in a total of 634 patients randomized to receive endovascular therapy in the five recent endovascular trials: one perforation in EXTEND IA, two in MR CLEAN, one in ESCAPE, five in REVASCAT, and one in SWIFT PRIME.1–5 Table 3 lists in a chronological order trials, registries, and case series with a reported incidence of vessel perforation from mechanical thrombectomy.1–5 ,8 ,9 ,13–16 It demonstrates a much improved safety profile of modern stent retriever technology over early generation thrombectomy devices.
Studies of mechanical thrombectomy with reported incidence of vessel perforation
By contrast, the findings of SAH on follow-up imaging in patients undergoing stent retriever thrombectomy are more common and its reported frequency ranges widely from study to study. This in part might be owing to difficulty in differentiating between true SAH and contrast staining, as well as the different definitions of hemorrhage used in each study. The literature suggests that SAH on postprocedural imaging recognized by an interventionalist during the procedure is rarely linked to a frank vessel perforation. For example, in a study of 74 consecutive patients with acute stroke treated with stent retriever thrombectomy, Yoon et al9 reported that 16% of cases showed either pure SAH or mixed SAH and contrast extravasation. No vessel perforation or intracranial dissections were reported in their cohort. The proposed mechanisms of such angiographically occult ruptures with extravasation of blood and/or contrast material included stretching of arterioles and accompanying venules in the subarachnoid space during the withdrawal of a stent retriever, as well as the disruption of cerebral microvascular permeability barriers.9 ,17 In the SWIFT and TREVO 2 trials, which compared the Solitaire (Medtronic) and Trevo (Stryker Neurovascular) stent retrievers with the Merci retriever device (Stryker Neurovascular), 4% and 11% of patients treated in the stent retriever arm were found to have SAH, but only one vessel perforation was reported in each trial, respectively.13 ,14
Such cases of postprocedural SAH without vessel perforation detected intraprocedurally are characterized by a rather benign course. In the study by Yoon et al,9 patients with SAH and those without had similar rates of good clinical outcome, based on discharge NIHSS and mRS scores at 3-month evaluations. Similarly, no increased risk for poor outcome in cases of stent retriever thrombectomy with imaging positive for hemorrhage plus contrast staining was shown in other studies.18 ,19
By contrast, our series included only cases where vessel perforation manifested by contrast extravasation was recognized immediately during the procedure. Our study indicates a rather malignant profile of this complication; 56% of patients died in the hospital, and the overall rate of poor outcome was 75% at 3 months. On the other hand, 25% of patients showed a favorable functional outcome, suggesting that some patients with intraprocedural perforation have a potential for good recovery. From our limited number of cases, we noted that bleedings which resulted in a pure SAH without formation of a parenchymal clot or hydrocephalus tended to have a more benign prognosis.
Several trends indicating which particular patients might be at a higher risk for vessel perforation can be seen in our study. First, there were a high number of patients with distal occlusions in our cohort (38%), whereas, on average, only 8–15% of patients undergoing stent retriever thrombectomy harbor a distal occlusion.20–23 Most perforations in our series occurred at distal sites, even in patients with occlusions involving more proximal vessel segments. Stent retrievers with a smaller diameter, such as the Mindframe Capture LP revascularization device (Medtronic) or the 3 mm Trevo device (‘baby’ Trevo, Stryker Neurovascular), were used in only three cases in our series. Such smaller profile devices might prove to be safer for occlusions involving more distal vessels, which have smaller diameter and more tortuous than proximal occlusions, as suggested by several studies.24–26
Second, in many cases, a ‘hard’ clot and difficulty traversing the occlusion were encountered. In only three of 10 cases resulting in distal perforation, direct aspiration with an intermediate catheter was attempted initially. One advantage of using the distal aspiration approach is that it does not require traversing the occlusion, thus, theoretically, reducing the risk of vessel perforation. Park and Kwak27 demonstrated an excellent safety profile of distal aspiration with a 4MAX Penumbra reperfusion catheter (Penumbra Inc) in a series of 22 patients harboring an M2 occlusion, reporting no direct vessel injury using this alternative approach to thrombectomy. For even more distal occlusions, such as the M3 MCA segment, a smaller profile aspiration catheter, such as the 3MAX Penumbra reperfusion catheter, can be used.
Publications dealing with specific rescue techniques for vessel perforations during stent retriever thrombectomy occur are lacking. Management of such complications generally follows the same principles as those that occur during endovascular treatment of aneurysms and other vascular lesions.28 Cone beam CT technology allows rapid assessment of the extent of ICH and recognition of herniation to determine if external ventricular drain placement is required without the need to transport the patient out of the angiography suite. Administration of protamine sulfate will rapidly reverse the systemic effect of heparinization. Infusion of platelets and cryoprecipitate is recommended for the reversal of the systemic fibrinolytic effect of tissue plasminogen activator, although its clinical benefits have not been proved.
Spontaneous resolution of bleeding can sometimes be observed after the device or microcather is withdrawn, because the clot itself can provide hemostasis at the site of vessel ruptured. For perforations that occur proximally to the occluded segment or are more extensive, inflation of an intracranial balloon for several minutes is often needed before hemostasis is achieved.29 The decision to proceed further or abort thrombectomy should be based on the severity of stroke and the extent of the residual clot. Unfortunately, even if subsequent thrombectomy is pursued and successful, delay in establishing reperfusion can result in a significant stroke burden. In cases where despite a repeat inflation of an intracranial balloon the bleeding continues, occlusion of the damaged vessel segment with embolic agents or detachable coils can be performed.30 Although certainly effective in achieving hemostasis, it will inevitably lead to a large stroke burden.
While preparing our series of cases, we came to realize differences among neurointerventionalists in their approach to thrombectomy cases complicated by vessel perforation. Some consider such cases as ‘hemorrhagic’ strokes and focus only on achieving hemostasis, while others consider successful reperfusion to be the ultimate goal and further attempt thrombectomy. Such differences in treatment strategies make it harder to interpret which rescue treatments have a better chance of providing clinically meaningful results. From our series, we could see that patients with a minor degree of hemorrhage, in whom at least a partial recanalization (TICI 2a or higher) was achieved, were more likely to have a good clinical outcome. Lack of any recanalization (TICI 0) was inevitably associated with death during hospitalization.
Other limitations of this study include its retrospective nature. Important technical details providing further clues to identify risk factors for this complication might have been missed. Ideally, such information should be gathered and analyzed from a prospective registry. Clinical trials also provide a very reliable and detailed data collection, but tend to exclude a large number of patients and, therefore, are not often reflective of real-world experience. For example, patients with distal MCA occlusions were under-represented in the recent randomized trials of endovascular therapy.1–5 Because this complication is (fortunately) quite rare, such a limited number of cases in our series precluded the use of statistical analysis to reliably determine which clinical or technical variables are associated with favorable outcome.
Conclusion
Intraprocedural perforations during stent retriever thrombectomy were observed in 1% of cases from our series, typically at distal vessel segments. Such cases were often characterized by difficulty traversing the occlusion with a microcatheter or microwire, and while withdrawing the stent retriever. Despite a number of attempted rescue approaches, the occurrence of intraprocedural perforations was associated with high mortality and low rates of good functional outcomes.
References
Footnotes
Contributors MM and KMF: study concept and design. MM: wrote the manuscript. MM and CTP: statistical analysis. All authors participated in data collection and analysis, edited the manuscript and approved the final version.
Competing interests AA: consultant—Covidien, Johnson and Johnson, Siemens, Stryker, and Terumo; grants—Siemens and Terumo. GD: consultant, proctor, speakers bureau—Medtronic, MicroVention; shareholder—Medina Medical, InNeuroCo, Stryker/Surpass. LE: consultant—Stryker Neurovascular, MicroVention, and Codman Neurovascular. RG: Grant-Zoll, WellStar foundation; honoraria—Penumbra, Inc; consultant/advisory board—Stryker Neurovascular, Covidien, Penumbra, Rapid Medical. PK: consultant—Stryker Neurovascular, Covidien, MicroVention. EIL: shareholder/ownership interests—Intratech Medical Ltd, Blockade Medical LLC, Medina Medical; principal investigator—Covidien US SWIFT PRIME Trials; honoraria for training and lecturing—Covidien; consultant—Pulsar, Medina Medical, Blockade Medical; other financial support—Abbott for carotid training for physicians. IL: consultant and speakers bureau—Covidien, Codman, Stryker. AHS: grants—National Institutes of Health/NINDS/NIBIB, University at Buffalo—none related to present study; financial interests—Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, and Lazarus Effect; consultant—Codman & Shurtleff, Inc, Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, Penumbra, Stryker, Pulsar Vascular, MicroVention, Lazarus Effect, Blockade Medical; speakers bureau—Codman & Shurtleff, Inc; national steering committee—Penumbra Incs 3D Separator Trial, Covidien's SWIFT PRIME trial, MicroVention’ s FRED trial; advisory boards—Codman & Shurtleff, Covidien Neurovascular; honoraria—Abbott Vascular, Codman & Shurtleff, Penumbra Inc. AST: consultant—Stryker, Codman, Penumbra, MicroVention; research grants (not related to present study)—Stryker, Codman, Micorvention, Penumbra, Covidien; financial interests—Medina Medical, Lazarus Effect, Pulsar Vascular, Blockade Medical.
Ethics approval Institutional review board at participating centers.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All data were presented in this paper.