Article Text
Abstract
Vessel wall magnetic resonance imaging (MRI) is a novel imaging technique that allows the intracranial vessel walls to be imaged directly. This state-of the art imaging modality may potentially change the way aneurysms are evaluated and managed. In this short review we discuss the current knowledge with illustrative cases.
- Aneurysm
Statistics from Altmetric.com
Introduction
In recent years advanced imaging techniques have become available that allow intracranial aneurysms to be assessed in ever greater detail. Some of these techniques are still in the research stage, but one technique is slowly entering clinical practice—namely, contrast-enhanced vessel wall MRI. This new imaging modality offers the potential to assess both unruptured and ruptured aneurysms at a pathological level and may provide further insight into this challenging condition. In this short review we present the current knowledge and demonstrate some of our own experience with this imaging method.
Wall imaging in unruptured aneurysms
The prevalence of aneurysms is approximately 3%1 across populations and they may cause significant stress and anxiety for patients. Large studies have investigated the rupture risk of these incidental aneurysms and found that several factors are important for predicting the risk of rupture, including aneurysm location, size, and morphology as well as gender, age, and ethnicity.2 The International Study of Unruptured Intracranial Aneurysms II investigators reported a threshold of 7 mm as a maximal diameter above which intervention should be considered.3 ,4 However, there is evidence that up to 37% of patients with subarachnoid hemorrhage (SAH) have aneurysms <5 mm in maximal diameter,5 and in the study of Ishibashi et al6 of 19 patients who had a rupture during follow-up, eight aneurysms were <5 mm and six had a maximum diameter of 3 mm. Therefore, this discrepancy between the current data must be resolved and a means of determining which aneurysms are at risk of rupture must be urgently sought.
Histopathological data have suggested that inflammation may play a role in the formation, growth, and rupture of aneurysms.7 Leukocyte invasion is commonly seen in ruptured intracranial aneurysms and numerous mediators of inflammation are involved.8 However, it is difficult to determine if this inflammatory cascade was active before the rupture or caused by the rupture itself. To demonstrate in vivo inflammation within the walls of aneurysms some investigators have used super-paramagnetic particles of iron oxide (ferumoxytol) as a contrast agent. They showed that circumferential wall uptake in an aneurysm wall obtained 24–72 h after infusion was highly predictive of aneurysm rupture within 6 months.9 It is worth noting that in this small study, 16 aneurysms were being actively observed and in this group three ruptures occurred. Each of these aneurysms demonstrated early enhancement and all were >15 mm in diameter.
Vessel wall MRI has been used to investigate enhancement in aneurysms. Edjlali et al used vessel wall MRI to evaluate both stable and unstable aneurysms. In unstable unruptured aneurysms—that is, those that were evolving in comparison with previous MR images or were symptomatic but not ruptured, circumferential aneurysmal wall enhancement (CAWE) was seen in 11/14 (79%) cases. In stable aneurysms, CAWE was seen in 22/27 (28.6%) cases and there was a statistically significant difference between stable and unstable aneurysms (p<0.0001).10 In the study of Nagahata et al strong enhancement was seen in 4/83 (4.8%) and faint enhancement in 11 (13.3%) aneurysms. Therefore, some enhancement was seen in 18%. Interestingly, two of the four unruptured aneurysms that showed strong enhancement were unstable, with one demonstrating rapid size increase and the other causing acute oculomotor nerve palsy. One of the remaining aneurysms was 3 mm and this exemplifies that this technique can show enhancement in very small aneurysms.11
Wall imaging in ruptured aneurysms
In cases of acute SAH the use of vessel wall MRI may prove extremely useful since up to 30% of patients may harbor more than one aneurysm.12 Treating an unruptured aneurysm in the presence of acute SAH may lead to complications related to the unruptured aneurysm (eg, aneurysm rupture), or to endovascular or microsurgical vessel manipulation resulting in worsening vasospasm, endothelial damage, or direct brain damage. Therefore, a dilemma exists about which aneurysm to treat. In most cases the clinical decision is based on the presenting CT scan, the appearance of any aneurysms (size, morphology, filling characteristics), and correlation of these data with the clinical history, if available.13 ,14
Matouk et al17 were the first to demonstrate the use of vessel wall MRI in patients with acute SAH. In their proof-of-principle study, they showed thick contrast enhancement of the aneurysm wall in all five patients. In three of these patients multiple aneurysms were present and enhancement was not seen in any of the unruptured aneurysms, although it is important to note that all three of these aneurysms were coiled and therefore, there was no pathological correlation.
However, surgical correlation between vessel wall enhancements has been found. In the series presented by Nagahata et al, 15 cases were seen to have enhancement at the apex or a bleb of the aneurysm. Of these 15 cases, seven underwent microsurgical clipping and in all these cases the rupture point correlated with the enhancement.11 Furthermore Kondo et al published a case of SAH in a patient with both an anterior communicating (ACOM) artery and a basilar tip aneurysm. In this case the basilar tip aneurysm was larger (6 mm) and therefore was thought more likely to be the source of the hemorrhage. However, vessel wall MRI showed thick enhancement of the smaller ACOM artery aneurysm. The patient was referred for microsurgical clipping, which directly identified the ACOM artery aneurysm as the source of bleeding.14
A recent case in our institution (figures 1⇓⇓⇓–5) had similar characteristics. A patient in their 40s presented with acute SAH (World Federation of Neurosurgical Societies grade 1). Diffuse subarachnoid blood was found on the initial CT scan and multiple bilateral aneurysms were found on CT angiography, the largest of which was on the left middle cerebral artery (MCA) bifurcation. Contrast-enhanced vessel wall MRI was performed and this showed thick CAWE of a smaller anterior communicating artery aneurysm and very faint enhancement of the largest left MCA bifurcation aneurysm. The patient was referred for microsurgical clipping as we could not reliably determine which aneurysm had bled. At surgery the ACOM artery aneurysm was confirmed to be the source of the SAH. After successful microsurgical clipping of the left MCA and ACOM artery aneurysms the patient made a full recovery (Extended Glasgow Outcome Score =8 at discharge).
(A) 3D digital subtraction angiogram. (B) Axial T1-weighted contrast-enhanced vessel wall MRI. A stable aneurysm on long-term (10 years) follow-up scans. The angiography was used to monitor another non-aneurysmal condition. Note the absence of enhancement seen on the post-contrast vessel wall MRI scan.
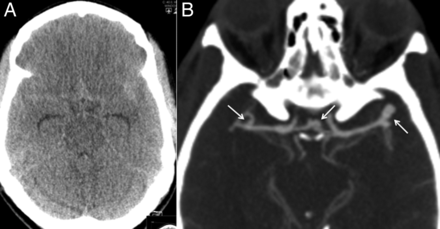
(A) Plain axial CT scan obtained about 12 h after the ictus. (B) Axial maximum intensity projection of a late arterial phase CT angiogram (100 mL Omnipaque). A patient in their 40s was admitted with acute subarachnoid hemorrhage. The blood was distributed diffusely with no pattern of localization. CT angiography was performed and this disclosed a large left middle cerebral artery (MCA) bifurcation aneurysm, a smaller anterior communicating artery aneurysm and small right MCA bifurcation aneurysm.
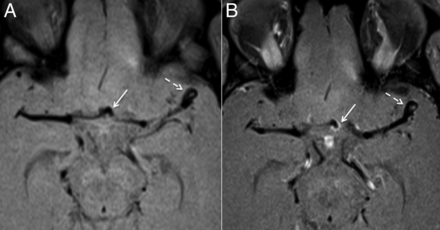
(A) Unenhanced axial vessel wall MRI. (B) Enhanced axial vessel wall MRI. Given the uncertainty as to which aneurysm had bled, the patient underwent dedicated vessel wall MRI. The MRI showed minimal contrast enhancement in the wall of the left middle cerebral artery (MCA) bifurcation aneurysm (dashed white arrows) but avid, thick enhancement of the anterior communicating (ACOM) artery aneurysm (solid white arrows). After discussion with the neurosurgical team, microsurgical clipping of the left MCA bifurcation and ACOM artery aneurysms was thought to be the most appropriate treatment.
Intraoperative photograph (obtained with a Zeiss Pantero 900 surgical microscope). The left middle cerebral artery (MCA) bifurcation has been fully dissected including the M1 and M2 branches. The aneurysm dome is fully exposed with no evidence of rupture. The MCA aneurysm was explored first as it was larger.
Intraoperative photograph (obtained with a Zeiss Pantero 900 surgical microscope). The left optic nerve is seen medial to the left internal carotid artery. The optic nerve runs into the chiasm to which the aneurysm adheres. The left A1 is fully exposed, as are both A2 arteries. The aneurysm neck arises from the right A1/A2 junction. The left recurrent artery of Heubner is seen arising from the left A1/A2 junction. Hemorrhagic brain was found adjacent to the aneurysm. As the clip was being deployed onto the aneurysm neck, the aneurysm ruptured again and this was controlled by fully deploying the clip. CN, cranial nerve; ICA, internal carotid artery.
These two cases highlight an important point—namely, that aneurysm size alone cannot be used to determine which aneurysm has ruptured when multiple aneurysms are present. Backes et al16 showed in their study of 124 patients with multiple aneurysms that the largest aneurysm had not bled in 29% of cases and, therefore, it is not unlikely that an unruptured aneurysm may be inappropriately treated.
To date the studies of Edjlali10 and Nagahata11 are the largest available. According to Nagahata et al, the presence of any wall enhancement yielded a sensitivity of 98.4% and specificity of 81.9% for the detection of aneurysmal rupture. These figures were 73.8% and 95.2%, respectively, if strong enhancement only was considered.11 Edjlali and colleagues did not calculate sensitivity and specificity from their data as they considered it might be inaccurate owing to referral bias and a likely disproportionate number of ruptured or symptomatic aneurysms. However, based on their presented data and accepting the potential for bias, their results yield a sensitivity of 87% and specificity of 71.4%.10
Conclusion
As technology continues to improve we find ourselves with an ever growing array of tools with which we must familiarize ourselves in order to provide the best care for our patients. Although vessel wall MRI is still in its infancy, it provides hope that aneurysms may be more readily assessed on a ‘pathophysiological’ level and not just on an ‘anatomic’ level. The technical requirements to perform vessel wall MRI are within the reach of many dedicated neuroscience centers and given the significant potential advantages of the technique, especially in cases of multiple aneurysms, it seems deserving of larger prospective studies.
Acknowledgments
The authors acknowledge Devika Ghisyawan, MRI superintendent radiographer, St Bart's and The Royal London Hospital, London.
References
Footnotes
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.