Article Text
Abstract
Intravenous tissue plasminogen activator has limited efficacy in fibrinolysis of large proximal intracranial thrombi. Thus, recent endovascular acute stroke trials restricted their selection criteria to patients with proximal occlusions in the anterior circulation. Although the terminal internal carotid artery occlusion is relatively easy to identify, there is often a debate as to what constitutes a proximal (involving the M1 segment) versus a distal (involving the M2 segment and beyond) middle cerebral artery occlusion. In light of overwhelming evidence demonstrating superiority of endovascular treatment in patients with proximal occlusion, this distinction has significant practical implications in patient selection. Here we present a brief review of the proximal (M1) segment of the middle cerebral artery anatomy and provide practical methods to recognize and separate the M1 and M2 segments. In keeping with the belief that CT angiography (CTA) (preferably multiphase CTA) is the ideal screening test for patients with emergent large vessel occlusion, we have provided tips for expeditious and accurate vascular imaging interpretation.
- Thrombectomy
- Stroke
- CT Angiography
Statistics from Altmetric.com
Background
Endovascular therapy is now the standard of care for acute ischemic stroke with occlusion of the M1 segment of the middle cerebral artery (MCA), with or without concomitant internal carotid artery (ICA) occlusion.1–5 In almost all cases determination of the occlusion site is made non-invasively by CT angiography (CTA) or MR angiography. This article aims to review the anatomical literature for the definition of the M1 MCA segment and to present a practical approach to quickly and accurately determine the site of occlusion on non-invasive vascular imaging.
There are many reasons why the recently published trials limited inclusion to patients with M1 MCA segment occlusions and excluded isolated M2 MCA segment occlusions. These are:
Data from previous studies showing that the recanalization rate of M1 MCA segment occlusions with IV tissue plasminogen activator is much lower than that of M2 MCA segment occlusions.6
M1 MCA segment occlusions have a much larger area at risk and hence, greater potential benefit of early recanalization. Correspondingly, the natural history of M1 occlusions is worse than that of M2 occlusions.
The lenticulostriate perforators that supply the basal ganglia usually arise from the M1 MCA segment; thus the ‘eloquence’ of the territory supplied by the M1 MCA segment is more pronounced than with the cortical M2 branches.
It is possible that the complication rate of endovascular treatment may be higher for the more distal M2 MCA segment occlusions than for M1 MCA segment occlusions.
A number of factors may contribute towards the fourth reason. First, current generations of stent-retrievers have generally been designed for the M1 segment; commonly used devices are 4 mm in diameter and 20 mm long. Second, there is often a significant curve (genu) just beyond the M1–M2 junction that influences the ease of retraction of the stentriever and has the potential to introduce complications. Third, the M1 often has a straight curve with small side-branches coming off at a steep angle making it unlikely for a stentriever to enter them. On the other hand, the M2 has a more tortuous curve and stentrievers may enter smaller side-branches, thus increasing the hemorrhagic risk. Also, M2 occlusions may be more challenging and time consuming as the neurointerventionalist has to selectively catheterize the correct occluded M2 segment. Clot retraction from the more distal cerebral circulation may make it increasingly difficult to transmit the suction pressure from the balloon guide catheter in the neck to the clot, thus decreasing the chances of successful thrombectomy and increasing the risk of non-target embolization. This may lead to increased use of large-bore distal access catheters.
Since many centers activate their neurointerventional teams based on the CTA-confirmed presence of a large vessel occlusion of the M1 segment of the MCA and/or ICA, it is critical that diagnosis of the occlusion is made quickly and that accurate distinctions are made between M1 and M2 occlusions.
Review of anatomy and embryology
The anterior cerebral artery (ACA) is the first and, phylogenically, the oldest telencephalic vessel. It can be considered the terminal branch of the ICA.7 During the first weeks of fetal life, when the embryo is 7–12 mm long, lateral striate arteries arise from its wall and provide blood supply to embryonic structures of the telencephalic vesicle that eventually become the MCA territory. Around the ninth gestational week, following the maturation of the vertebrobasilar system, the MCA develops from the fusion of several ACA perforators of the lateral striate group. This developmental pattern matches that of the recurrent artery of Heubner (RAH). Since both the MCA and the RAH share the same phylogenetic origin, their territories and anatomical variations are related to each other. Both vessels give rise to perforating centripetal corticostriatal branches supplying, among other territories, the medial putamen, the caudate, and part of the anterior limb of the internal capsule.8 ,9
MCA segment branches
Lenticulostriate perforators
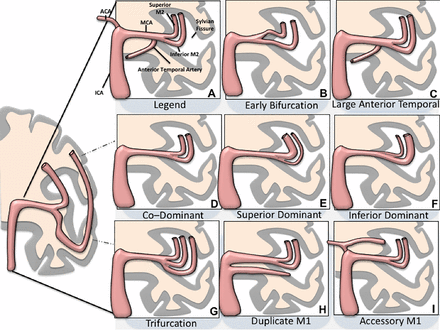
There is a broad range of variations related to the perforators owing to differing coalescence during embryonic life of the different perforator groups. These perforators may arise as multiple single small vessels from the parent artery or from a common larger trunk or take over each other's territory. The MCA typically gives rise to the medial and lateral lenticulostriate artery groups.7 ,9 These groups can be interconnected and form a rete (the most prominent example of which is the fenestration of the M1). They are in balance with the lenticulostriate arteries arising from the ACA, an equilibrium which mainly involves the medial group. There are usually between 6 and 20 lenticulostriate branches for each hemisphere that may arise as single vessels or, more commonly, from two or more major trunks (so-called candelabra arteries), which then divide into smaller ones.10 Most commonly they arise from the posterosuperior aspect of the M1 segment and, in rare instances, they originate from either the superior or inferior divisions of the MCA or, even less commonly, from an early cortical branch of the MCA.10 A schematic representation of a variety of M1 MCA segments with branches is shown in figure 1.
Schematic representation of a variety of middle cerebral artery (MCA) variants, as represented on a coronal image of the brain. ICA, internal carotid artery.
First order branches
Distal to the MCA trunk, the MCA typically splits into two divisions in about three out of four people. These superior and inferior trunks are in hemodynamic balance and vary considerably in the dominance of one trunk over the other (with concomitant annexed territories).11 The superior division will supply the frontal convexity, whereas the inferior trunk will supply the temporal lobe. The parietal lobe may be annexed by either trunk and this establishes dominance. In patients with a bifurcation, co-dominance is seen in 18%, superior division dominance in 28%, and inferior division dominance in 32%. An anterior temporal branch can arise proximally from the MCA trunk and should not be confused with an early bifurcation of the M1 (figure 2). Less commonly, the MCA will split into three divisions (12%) or more than three division (10%).8
(A) Coronal maximum intensity projection (MIP) reconstruction from a CT angiogram (CTA) demonstrates the normal anterior temporal artery (arrow), arising in its usual position. Note how the size of the M1 segment remains constant before and after the origin of this small branch. Note the occlusion of the distal right M1 segment and complete occlusion of the left internal carotid artery. (B) Axial MIP image from a CTA. In contrast to the previous image, notice how this patient has an early bifurcating M1 segment (arrow), with a caliber change as the M1 splits into two M2 branches.
In the usual ‘textbook’ anatomy, the superior and inferior divisions are co-dominant: in these cases the superior branch would supply the orbitofrontal; prefrontal; precentral and central vascular territories, whereas the inferior division would give rise to the parietal; angular; temporo-occipital, posterior temporal; middle and anterior temporal vascular territories. As discussed above, depending on the individual anatomy, however, any type of anatomical variation can be found, including a dominant superior branch that annexes the parietal or even angular arteries, and a dominant inferior branch that gives rise to central and even precentral arteries (figure 3).11 In these latter cases a single inferior branch may be overwhelmed by the territory it has to supply. Thus, during embryological pruning a temporal branch that will give rise to posterior, middle, or anterior temporal branches can be present.11 Finally, the anterior temporal artery may arise as a single vessel early from the M1.12 The discussion about where the M1 stops and where the M2 starts is therefore an artificial one, necessitating a more ‘practical’ approach for everyday work.
(A) This coronal maximum intensity projection (MIP) image from a CT angiogram demonstrates the concept of ‘dominance’, with a dominant inferior division of M2, which is the most common branching pattern (64–90%). (B) On sagittal MIP images from the same patient, we can see that this inferior division is the dominant supply to middle cerebral artery opercular branches.
Second order branches
The distinction between M2 and M3 MCA segments is made not on the site of the next bifurcation, but rather based on an exit from the Sylvian fissure (figure 4). The distal (M3 and M4) MCA branches are named, irrespective of their origin, but rather based on the cortical territory they supply, which leads to the following MCA ‘branches’: orbitofrontal; prefrontal; precentral (preRolandic); central (Rolandic); anterior parietal; posterior parietal; angular; temporo-occipital, posterior temporal; middle and anterior temporal. These branches are best appreciated on the lateral view.
Distinguishing M2 from M3 branches depends on the location of the branches. On this coronal MIP image from CT angiogram (same image as figure 1), the location of the M2 segments (vertically oriented, dashed circle) is highlighted.
Practical considerations for an expeditious determination of site of occlusion
Format for reviewing the imaging
Most often determination of a proximal M1 occlusion depends on the way in which the data are presented. Since the source images of CTA or MR angiography are available before the reformats, it is reasonable to look at the source images as soon as they are available to save time. However, if a distal M1 occlusion (or possibly an M2 occlusion) is present, it may be worthwhile examining the reformats, especially the thick-section multiplanar reformats or maximum intensity projections. These are typically the ‘money images’ for looking at large vessel occlusions. If the occluded vessel looks smaller than the usual size of M1, an attempt should be made to look for another branch. We find that coronal reformats are most useful in this regard.
The anterior temporal artery
Early M1 branches are those that arise proximal to the M1 division. The largest of these early branches is the anterior temporal artery that typically arises from M1 mid-segment but can arise from an M1 trifurcation or an M2 branch. Many authorities agree that from a practical standpoint a small anterior temporal artery is considered a branch of M1 (and not M2). This raises the question: what is small? Should this determination be made on absolute size, or on the ratio between the sizes of the anterior temporal artery relative to the proximal M1? Alternatively, should it be based on following the vessel to determine approximately the part of the brain being supplied by this vessel? One practical way to think about the problem is as follows: if the artery looks like an anterior temporal branch but exits the Sylvian fissure and supplies territory beyond the anterior temporal lobe (including the posterior temporal or low parietal regions) it may be considered an M2 MCA segment rather than an anterior temporal artery. Thus, in practical terms, a large artery that supplies other vascular territories in addition to the anterior temporal artery should be considered an M2 MCA segment for operational reasons. The reader may trace the arteries with CTA or use the third phase of a multiphase CTA scan (area of delayed washout) to make this determination. Perfusion maps can also be used to discriminate between an MCA M1 segment occlusion that is distal to an anterior temporal artery branch and a similar occlusion that is in an M2 MCA segment. In patients with an M2 occlusion, the perfusion delay/defect will only involve part of the traditional MCA territory (figure 5). However, this is dependent on the imaging protocol and many centers may not have whole brain coverage as part of their CT perfusion protocol.
(A) The utility of perfusion maps (when performed) in finding an M2 occlusion. Multiple coronal maximum intensity projection reconstructions from a CT angiogram demonstrate left M2 division occlusion (thin white arrow). This patient has a patent normal left anterior temporal artery (red arrow) and patent superior division (inset image). (B) Axial cerebral blood flow maps from the same patient demonstrate an area of diminished perfusion involving a portion of the left middle cerebral artery territory, suggesting an M2 branch occlusion.
Very early bifurcation of the M1 MCA segment
It is important to recognize anatomical variants, including duplicated M1 MCA and the accessory MCA (see figure 1 for a pictorial description of all variants). These may be overlooked on axial images but are often more readily apparent on coronal reformats. Often, the two M2 branches will be similar in size. From a practical perspective, knowledge of this anatomy helps. In addition, tracing the arteries concerned by CTA, using the third phase of a multiphase CTA scan or a perfusion-weighted image, can assist in making a quick decision.
Occlusion of both/all M2 divisions (a functional M1 or M1 equivalent)
Some trials have allowed M2 divisions to be included. It should be noted, however, that these can present a technical challenge for the neurointerventionalist as each of the occluded M2s has to be opened individually. Fortunately, this circumstance is rare.
Use of the clinical examination
In many cases, the degree of clinical deficit (as measured by the National Institute of Health Stroke Scale (NIHSS)) can be a helpful surrogate marker. Prior studies have shown a correlation between the site of occlusion and degree of clinical deficit.13 This can be helpful in cases where there is a large clinical deficit (ie, NIHSS ≥10), but the occlusion is apparently in an ‘M2’, when it is actually an M1 occlusion. Detailed review of the CT angiogram may show that the occlusion is really in an M1, or suggest variant anatomy, as described above.
Conclusion
We have presented a brief review of proximal MCA anatomy, the reasons why trials exclude M2 occlusions, practical problems in recognizing and separating M1 from M2 and some tips to allow for expeditious interpretation of imaging and avoid errors and protocol violations. It is possible that occlusion of a large M2 vessel (based on a large clinical deficit, large size of occluded vessel, and possibly, a large perfusion defect) might behave like an M1 occlusion and might benefit from endovascular treatment. However, these patients were not represented in the recent wave of endovascular trials, and future trials are needed to verify this hypothesis.
References
Footnotes
Contributors All authors made substantial contributions to the conception or design of the work; were responsible for drafting the work or revising it critically for important intellectual content; had final approval of the version to be published; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.