Article Text
Abstract
Introduction Wide-necked bifurcation aneurysms (WNBAs) present unique technical challenges for both endovascular and surgical treatments which aim to achieve complete occlusion of the aneurysm without compromising the patency of the incorporated regional parent vessels. We present a meta-analysis of traditional therapies for WNBAs to provide critical benchmarks for safety and effectiveness.
Methods Following a systematic search of the literature and the application of pre-specified appropriateness criteria, 43 (including 2794 aneurysms treated) and 65 (including 5366 patients treated) references with sufficient detail were identified to include in a meta-analysis of efficacy and safety, respectively. Effectiveness endpoints of both complete and adequate occlusion were assessed. A composite safety endpoint was based upon commonly applied metrics for major adverse events. Fleiss analyses were performed for both effectiveness and safety endpoints for the entire group, and then parsed separately by treatment modality (surgical clipping (SC) or endovascular therapy (EVT)) and location (anterior or posterior circulation).
Results Using the above methods, the core laboratory adjusted rate of complete occlusion was 46.3% (standard error 3.6%), 39.8% (3.7%), and 52.5% (9.6%) for all therapies, EVT, and SC, respectively. The rate of adequate occlusion was 59.4% (12.2%), 43.8% (5.3%), and 69.7% (14.3%) for all therapies, EVT, and SC, respectively. The rates of occurrence for pre-specified safety endpoints were 18.7% (2.9%), 21.1% (2.8%), and 24.3% (4.9%) for all therapies, EVT, and SC, respectively.
Conclusions Conventional therapies for WNBAs are associated with relatively low rates of complete occlusion and peri-procedural complications are not uncommon. As new treatment technologies are investigated, it is important that the available data regarding predicate treatments is understood.
- Aneurysm
- Hemorrhage
- Intervention
Statistics from Altmetric.com
Introduction
Wide-necked bifurcation aneurysms (WNBAs) present specific technical challenges for existing endovascular and surgical treatment strategies. A number of novel devices have been developed to address this unmet clinical need. The design and analysis of clinical studies of such devices requires the demonstration of equivalence to established therapies. In order to determine the true relative safety and effectiveness of these new devices, the definition of benchmark parameters for market-approved comparators is essential.
To this end, we present a comprehensive meta-analysis of studies describing the safety and effectiveness of both conventional surgical and endovascular treatment (EVT) strategies for WNBAs. These data may ultimately serve as Objective Performance Criteria (OPC) for the evaluation of novel wide-necked bifurcation intracranial aneurysm devices.
Methods
Literature search
In conducting this meta-analysis, the authors subscribed to a PRISMA-P approach (Preferred Reporting Items for Systemic Review and Meta-Analysis Protocols).1 Systematic searches of PubMed were conducted, using preset search terms, to find peer-reviewed articles reporting on the safety and/or effectiveness outcomes following surgical treatment and EVT for wide-necked bifurcation intracranial aneurysms (figure 1). Employing EndNote X7.7.1 to import the results, ‘All Fields’ were searched for ‘intracranial aneurysm’ or ‘cerebral aneurysm’ or ‘brain aneurysm’. This initial search yielded 34 531 articles, with duplicates removed (Step 1). After removing articles published prior to 1 January 2000 in Step 2, a total of 18 738 remained for further consideration. This date was decided on prospectively, based on the introduction of three-dimensional Gugliemi detachable coils for the treatment of wide-necked aneurysms in 1999 and the general availability of EVT by that date.
Flowchart of literature search and Objective Performance Criteria (OPC) calculation process.
Our familiarity with the intracranial aneurysm literature provided the background knowledge that intracranial aneurysm publications reporting on WNBAs may not be identified by searching one specific keyword or key phrase. That given, the remaining articles were filtered for wide-neck and for bifurcation aneurysms (multiple word variations included). The resulting wide-neck subgroup contained 788 articles while the bifurcation subgroup yield was 816 articles (Step 3).
Following the next steps, the goal was to create two pools of articles with potentially adequate data for analysis—one for effectiveness outcomes (152 articles) and the other for safety outcomes (189 articles). Exclusion criteria (Step 4a) were applied to both subgroups, limiting the number of studies moving forward. For this analysis, exclusion criteria were the same for both article subgroups. Sound statistical practice requires controlling for selection bias within articles and heterogeneity both within and across studies. The following exclusion criteria were established to limit these sources of error and variance:
case series with fewer than eight subjects
series in which wide-necked bifurcations constituted less than 65% of aneurysms treated
studies with a follow-up rate of less than 65% of the treated population
Other criteria were designed to exclude studies of treatment modalities outside of established technologies—namely coil, stent and coil, and open surgical clipping. For the specific purpose of this meta-analysis, publications were limited via additional exclusion criteria to studies of the following types:
adult human populations predominantly treated for a new (not recurrent) small, medium, or large saccular WNBA
implanted device(s) were used in accordance with the indications for use (ie, not off-label)
study population did not include the same subjects as another study (to avoid double-counting)
study population was not from a national database or registry that may have a subpopulation from another study (again to avoid double-counting)
language of publication was English or an English version was obtainable.
Inclusion criteria for safety and effectiveness were specified to achieve a comparable dataset across studies of WNBA treatment (Step 4b). For effectiveness, the inclusion criteria required that all studies report both initial imaging results and at least one follow-up image at 12 months (range 4–25 months) post-procedure that was reported according to a recognized occlusion scale such as the Raymond/Montreal scale or percent occlusion. Furthermore, each article was required to report an identifiable population or subpopulation of either wide-necked or bifurcation or WNBAs.
Safety inclusion criteria (also Step 4b) similarly required that candidate articles report on an identifiable population or cohort of wide-necked aneurysms or bifurcation. These criteria also defined the following requisite minimums for clinical outcomes data reporting at the initial, post-procedural (within 30 days of procedure), and 6 and/or 12 months follow-up as defined below:
report of deaths peri-procedurally and at all follow-ups above and
reported at least one ischemic safety endpoint (ischemic stroke, TIA) and/or
reported at least one non-ischemic safety endpoint (hemorrhagic stroke, thromboembolic event, intracranial bleed, vasospasm, ischemic neurologic deficit or parent artery occlusion) and
6-month clinical outcome, defined as actual or mean follow-up at 6 months (range 4–9 months) and/or
12 months clinical outcome (range 10–25 months)
In Step 5, additional search methods were employed (online Supplementary appendix 1 for a complete list) to complement the yield of Step 1. These methods included online search engines, relevant journal articles, and the evaluation of references cited within previously identified publications. Measures were also taken to account for articles published between the initial search date and the data lock (30 September 2016) using the ‘My NCBI’ tool in PubMed and the Google ‘Alerts’ function of Google Scholar to monitor daily for articles fitting the Step 1 search criteria. Articles found via any of these approaches were subjected to the same Steps 2–4b and three more articles were identified for consideration, increasing the effectiveness and safety article pools to 154 and 190 unique articles, respectively.
Supplementary file 1
The last step prior to data analyses was Step 6, readying the final outcome data for analysis. Following comprehensive review of the unique 154 effectiveness articles and 190 unique safety articles remaining after Step 5 (Supplementary appendix 2), the final pools of literature for calculation of effectiveness and safety rates were created based on the following final criteria:
Aneurysm presentation and aneurysm post-treatment outcomes were reported by aneurysm location (anterior vs posterior) and
Subgroups with eight or fewer subjects treated in a given location subset were deleted from the analysis
Only outcome data at 12 months postoperatively were used in the final statistical analysis (when only 6-month outcome data were available the 6-month outcome was ‘carried forward’ and used as the surrogate 12-month outcome)
Multiple references informing on two Level 1 evidence studies—the Cerecyte Coil Trial (CCT) and the Matrix and Platinum Science trial (MAPS)—were included in the final literature pool.2–5 While not all presented a full complement of outcome data, used collectively and filtered for wide neck bifurcation data only, they provided two populations highly relevant to this analysis. Personal communications with Dr Molyneux confirmed that the Cerecyte Coil Trial Wide Necked Bifurcation Aneurysm Dataset (CCT WNAD) could be employed in this analysis as a stand-alone Level I reference.4 The CCT WNAD was unpublished patient level data that provided a wide-necked bifurcation aneurysm (WNA) subset from the Cerecyte Coil Trial. Data from the MAPS trial were narrowed to bifurcation WNAs only (found in Hetts et al and informed on by McDougall et al) to provide a corresponding dataset to the CCT WNAD.2 5
The distribution by aneurysm location of the final pool of 43 effectiveness references (42 articles + CCT WNAD, 2794 aneurysms) was as follows:
31 effectiveness references (1749 aneurysms) reported on anterior aneurysms (30 articles + CCT WNAD)
25 effectiveness references (1045 aneurysms) reported on posterior aneurysms (24 articles + CCT WNAD)
The distribution by aneurysm location of the final pool of 65 safety articles (5366 patients) was as follows:
53 safety articles (3553 patients) reported on anterior aneurysms
33 safety articles (1813 patients) reported on posterior aneurysms.
For each of the final 43 effectiveness articles, both complete occlusion (Raymond 1) and adequate occlusion (Raymond 1 and 2) effectiveness outcome data were identified for analysis. For the final 65 articles included in the safety analysis, the following outcome data were tabulated: major stroke, ipsilateral stroke, new subarachnoid hemorrhage (SAH), rebleed SAH from the target aneurysm, significant parent artery stenosis, significant vasospasm, neurological death, and non-neurological death.
Search limitations
From the analysis of this literature search it is clear that substantial variation exists in the percentages of aneurysms that attain a complete occlusion outcome at 12 months. Factors influencing this outcome measure include but are not limited to the following points:
For some studies, morphology of aneurysms is not exclusively WNA at the bifurcation (thus, aneurysm locations for the published study were not isolated to the targeted locations criteria).
Assessment of occlusion success was done by the investigator rather than a core laboratory in the vast majority of studies.
‘Survivor effect’—bias introduced by which subjects presented for follow-up and how the investigator accounted (or did not account) for those who did not present—cannot be eliminated from the analysis.
Treatment with adjunctive implants may have improved the effectiveness outcome for a given subject/aneurysm.
Data synthesis and analysis
In Step 7, Fleiss analyses were performed for both effectiveness and safety study rates and standard errors (SEs) by aneurysm location.6 Details of these calculations are presented below.
Calculation of effectiveness study rates and SEs by locations
Because most of the retrospective studies did not employ an independent core laboratory for angiographic evaluations, a means of outcome data correction must be established in order to make core laboratory data comparisons. To determine the appropriate adjustment, three recent studies provided estimates of the difference between angiographic outcomes as assessed by core laboratories and those assessed by individual operators. In a study published in 2014, Rezek et al found an absolute 26% lower rate of complete occlusion outcomes between core laboratory and operator-read images in the primary endpoint of the 433-patient Cerecyte Coil Trial.7 Taki et al reported a 24% lower rate of complete occlusions from core laboratory assessments compared with investigator assessments in the prospective 129-patient PRESAT study.8 Finally, in a separate meta-analysis of studies published between 1999 and 2011, in 2013 Rezek et al reported an absolute 7.1% lower unfavorable outcome rate in 8 of the 104 studies reviewed.9 These three studies were used to calculate a core laboratory correction factor.
Although Rezek et al looked at the rate of incomplete occlusion rather than complete occlusion in their 2013 study, their results were a mean derived from eight core laboratory studies whereas the other two studies reported on a single core laboratory study.7 This difference should result in a fourfold higher weight for the results of the 2013 study by Rezek et al compared with the studies of Taki et al and the 2014 study by Rezek et al.7–9 However, unlike Taki et al and the 2014 study by Rezek et al, in their 2013 study Rezek et al did not report the total numbers of aneurysms evaluated.7–9 Thus the rate reported in the 2013 study by Rezek et al was assigned three times the weight of the rates of the studies by Taki et al and the 2014 study by Rezek et al.
The results of the non-core laboratory literature studies above should be down-weighted by the value obtained from the formula:

To be conservative, this was rounded down to 12%. The Core Laboratory Adjusted Rate is calculated as:

The calculated Core Laboratory Adjusted Rate is rounded to the nearest whole number and used to report the adjusted number of totally or adequately occluded aneurysms for each study. It is important to note that no adjustment was applied to the studies that employed a core laboratory.
Study rates were derived by combining studies for each location (anterior and posterior) and treatment modality (EVT and surgical) by the inverse variance weighting Fleiss method with adjustment for non-homogeneous rates.6 The adjusted numbers of complete occlusion and adequate occlusion were independently combined into a single estimate by weighting each literature estimate (Yc) by the inverse of the variance of the estimate (Wc) by the Fleiss method.6 This method is necessary because the anterior rates vary from 18.6% to 78.3% and the posterior rates vary from 25.0% to 91.7%. The Fleiss method was used to compute 95% confidence limits for unadjusted and adjusted combined rates with consistent theoretical justification.6
The estimates were obtained by dividing the sum of the YcWc divided by the sum of the Wc. Because Q was greater than the number of studies minus one, there was evidence of inhomogeneity among the rates for the studies cited and a further adjustment was made, resulting in a rate of 0.1846 with a SE of 0.1218.
In the same analysis of the surgical data, the posterior location had only a single study. There was no need to use the Fleiss method for a single study because there was no combining of study results. The results of the only posterior study were used along with the anterior circulation clipping results in the calculation of a surgical effectiveness OPC.
Results from the analysis of the EVT and surgical treatment modalities are combined using the same Fleiss method.
Calculation of safety study rates and SEs by locations
The data were compiled by location and time. In a number of studies, multiple events for a given patient were reported and more events than patients enrolled were also described. It was also noted that, in studies with patient-by-patient data, one in eight major strokes resulted in a neurological death (strokes and death occurred in the same follow-up time period). Safety data therefore required adjustment to correct for multiple events in the same patient, as only one clinical outcome per subject was permitted. This adjustment was accomplished in the following ways: (a) for all studies that reported multiple events for a given patient, that patient was assigned only the most serious event; and (b) for all studies reporting neurological death and a safety event (as defined in Step 6), only the neurological death was counted.
After the adjustments itemized above, the rates across the 12 months of follow-up were computed and combined by the method of Fleiss for the locations and modalities of interest. The rates often varied among the device types, requiring a further adjustment proposed by Fleiss to account for inhomogeneity among the rates.6
Calculation of the overall study rate and SE by modality
Since the study rates were calculated for anterior and posterior locations separately, the overall estimated study rate from the literature for a given modality was calculated based on the proportion of anterior and posterior aneurysms included in each literature pool. Therefore, in Step 8 the SE of this rate was obtained by the formula below:

Calculation of the OPC
The OPC (Step 9) was derived directly from the study rate  and the SE. Next, the OPC for effectiveness was calculated as the lower 95% confidence limit for literature-derived study rate using the following formula:
and the SE. Next, the OPC for effectiveness was calculated as the lower 95% confidence limit for literature-derived study rate using the following formula:

Conversely, the OPC for safety was calculated as the upper 95% confidence limit for literature derived study rate using the following formula:
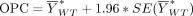
Results
Efficacy
The Fleiss analysis for the study rates and SEs, along with the OPC for the observed and core laboratory adjusted numbers of aneurysms at 12 months for each modality and location, were calculated separately for totally occluded aneurysms and adequately occluded aneurysms. The results of the analysis are presented in table 1.
Rates of aneurysm occlusion in meta-analysis
Using the above methods, the core laboratory adjusted complete occlusion rates were 46.3% (SE 3.6%), 39.8% (3.7%) and 52.5% (9.6%) for all therapies, EVT, and SC, respectively. Efficacy rates for adequate occlusion were 59.4% (12.2%), 43.8% (5.3%) and 69.7% (14.3%) for all therapies, EVT, and SC, respectively. When only Level I studies are considered, however, the rates of complete occlusion were substantially lower: 34.9% (5.7%), 28.7% (7.7%), and 43.5% (3.4%) for all therapies, EVT, and SC, respectively.2 10–12
Looking at rates of occlusion based on anatomical distribution of aneurysms, the rates of complete occlusion were 43.7% (4.7%) and 50.6% (5.6%) for anterior and posterior circulation aneurysms, respectively, when all therapies were considered. When EVT alone was considered, the rates of complete occlusion were 41.7% (4.6%) and 37.5% (6.2%), respectively, for anterior and posterior circulation aneurysms. Rates of adequate occlusion were 60.3% (19.6%) and 58.1% (7.9%) for all therapies in the anterior and posterior circulation distributions, respectively. When EVT alone was considered, rates of adequate occlusion were 41.8% (4.6%) in the anterior circulation and 45.9% (10.1%) in the posterior circulation.
The calculated OPC for efficacy (complete occlusion) were 39.3% and 32.5% for all modalities and EVT, respectively.
Safety
A safety endpoint occurred within 12 months of treatment in 18.7% (2.9%), 21.1% (2.8%), and 24.3% (4.9%) for all therapies, EVT, and SC, respectively.
When evaluation of Level I studies is considered, rates of safety events were higher with 48.5% (13.0%), 40.3% (21.6%), and 66.8% (2.7%) for all therapies, EVT, and SC, respectively.2 12
When parsed by anatomical location, safety events occurred during the treatment of 18.4% (3.2%) and 19.3% (6.0%) of anterior and posterior circulation aneurysms, respectively, when all therapies are considered collectively.
The calculated OPC for safety were 24.5% and 26.7% for all modalities and EVT, respectively.
Discussion
Any assessment of new technologies for the treatment of WNBAs is predicated on a comparison with existing data. The existing body of data for WNBA, while certainly limited, documents that conventional treatments result in relatively low rates of complete (~45%) and adequate occlusion (~60%) and relatively high rates of complication as assessed by safety endpoints (~19%). These data support continued efforts to develop new treatment strategies for these lesions.
Given the marked variation in potential treatment strategies for these aneurysms and the lack of an accepted ‘gold standard’ approach, it is impractical to design a concurrent control arm for a randomized trial assessing new technologies in this space. Until and unless a more uniform, definitive treatment becomes established, novel devices and approaches will, by necessity, continue to be evaluated within the context of single-arm studies in which outcomes will be compared with literature-based OPC. The OPC described herein may be used to establish the non-inferiority of novel technologies and treatment strategies as they evolve.
It must be acknowledged that, despite the application of fairly rigorous criteria for the selection of relevant studies, the existing body of literature has many limitations. There is a paucity of Level I data and a large number of retrospective case series which, in some cases, included results from only a single center. Outcome measures were often inconsistent and, in some cases, methods of assessment were poorly described. Many studies used operator-adjudicated, non-core laboratory-assessed, and unblinded outcome measures for efficacy and safety. To adjust for the well-documented bias introduced by self-adjudication, a ‘core laboratory’ adjustment was calculated based on existing neurovascular studies that compared core laboratory with self-adjudicated outcomes. This ‘core laboratory’ adjustment was applied to all non-core laboratory-adjudicated data in an attempt to account for this bias systematically. Moreover, many studies evaluated ‘wide-necked aneurysms’, a proportion of which were sidewall aneurysms. When possible, bifurcation aneurysms were segmented out of these datasets, as described in the Methods section. However, the safety and efficacy calculations still very likely incorporate a small percentage of wide-necked sidewall aneurysms from some datasets without a complete anatomical segregation. Finally, WNBAs represent a relatively heterogeneous group of lesions with respect to aneurysm size, anatomical location, morphology, and the configuration/incorporation of the vessels constituting the bifurcation. In addition, treatment strategies, particularly endovascular strategies, have rapidly evolved. These factors resulted in a high level of treatment variability over time, across operators, and between centers. For these reasons, considerable heterogeneity exists between the datasets from the available studies and SEs associated with the datasets are relatively high. Additional assumptions and their associated limitations which pertain directly to the literature search performed are addressed in the Methods section.
These data support the longstanding conventional thinking for aneurysm treatment in general, in that surgical clipping yielded higher rates of complete occlusion than EVT. However, the higher rates of complete occlusion achieved with surgical clipping came at the expense of a higher incidence of safety endpoints. The incidence of safety endpoints and the absolute differences between modalities were more pronounced in the Level 1 studies than in the overall body of literature.
Conclusions
Conventional therapies for WNBAs are associated with relatively low rates of complete occlusion and peri-procedural complications are not uncommon. Newer therapies are needed for the treatment of these aneurysms. Until a ‘gold standard’ treatment strategy is established, these emerging technologies can be evaluated in comparison to the benchmark literature-derived OPC presented.
Supplementary file 2
References
Footnotes
Contributors All named authors contributed substantially to the work described by actively participating in the study and the generation of the data and providing editorial evaluation of the manuscript.
Funding Statistical support for this study was provided by Microvention Inc.
Competing interests The primary investigators received institutional salary support for the WEB-IT study, proctoring, consulting and study-related activities from Microvention Inc.
Provenance and peer review Not commissioned; externally peer reviewed.



