Article Text
Abstract
Objective Evaluation of the safety and efficacy of the Pipeline embolization device (PED) when used as second-line treatment for recurrent or residual, pretreated ruptured and unruptured intracranial aneurysms (IAs).
Methods Retrospective review of our database to include all patients who were treated with a PED for recurrent or residual IAs following surgical clipping or coiling. We evaluated neurological outcome and angiograms at discharge, 6- and 12-months’ follow-up and assessed intimal hyperplasia at follow-up.
Results Twenty-four patients met our inclusion criteria. Most IAs were located in the anterior circulation (n=21). No change of preprocedure modified Rankin Scale score was seen at discharge or at any scheduled follow-up. Complete or near-complete aneurysm occlusion on 6- and 12-month angiograms was seen in 94.4% (17/18 cases) and 93.3% (14/15 cases), respectively. Complete or near-complete occlusion was seen in 100% of previously ruptured and 85.7% (6/7 cases) and 83.3% (5/6 cases) of previously unruptured cases at the 6- and 12-months’ follow-up, respectively. One case of moderate intimal hyperplasia was observed at 6 months and decreased to mild at the 12-months’ follow-up. No difference in device performance was observed among pretreated unruptured or ruptured IAs.
Conclusions Treatment of recurrent or residual IAs with a PED after previous coiling or clipping is feasible and safe. There is no difference in device performance between ruptured or unruptured IAs.
- Flow Diverter
- Aneurysm
- Coil
- Intervention
Statistics from Altmetric.com
Introduction
Introduction of flow diversion (FD) technology to the neuroendovascular realm represents a paradigm shift for the treatment of intracranial aneurysms (IAs). IAs previously categorized as ‘difficult’ to treat are now often amenable to FD. These devices have been designed for reconstruction of the parent vessel and progressive occlusion over time, secondary to blood stasis within the IAs.1 Flow diverters show high rates of aneurysm occlusion with low recurrence and re-treatment rates.2 ,3
Long-term success rates of coiling with complete obliteration are about 50–85%, dependent on size and location of the IAs. Aneurysm recurrence after coiling is reported in up to 50% of patients4–6 and is due to coil compaction, incomplete aneurysm neck scarring, or aneurysm regrowth. IA re-treatment rates range from 4.7% to 10%.7–11 IA residuals after surgical clipping are seen in 4–8%.12–14 and carry a persistent risk of regrowth and (re)rupture.
The current literature provides little information on the performance of FD when used as second-line treatment in patients with recurrent or residual cerebral aneurysms, especially in previously ruptured cases. The goal of this single-center study was to assess the efficacy and safety of the Pipeline embolization device (PED) in the treatment of recurrent or residual IAs and to evaluate its performance in unruptured and ruptured previously coiled or clipped IAs.
Materials and methods
This retrospective study was approved by our hospital institutional review board.
Between October 2011 and July 2016 we treated 173 IAs with the PED at our institution. All recurrent or residual IAs were identified that had been previously treated with either coiling or surgical clipping. Information was gathered on patient demographic data, including vascular risk factors, age, gender, rupture status, and IA size and location.
Patient neurologic examination based on the modified Rankin Scale (mRS) score was available before the procedure, at discharge, and at 6- and 12-months’ follow-up. Degree of aneurysm occlusion (modified Raymond scale), parent vessel patency, and presence of intimal hyperplasia (IH) were evaluated on 6- and 12-month follow-up angiograms.
Results
Patient and aneurysm characteristics
We identified 24 patients with 24 IAs who had been previously treated with either coil embolization (n=18) or surgical clipping (n=6). Twenty subjects showed recanalization while four cases were residual aneurysms. Patients' mean age was 54.5 years (range 20–71 years) and seven patients were male. Vascular risk factors included hypertension (62.5%), smoking (45.8%), dyslipidemia (29.2%), and diabetes (8.3%). Fourteen of the aneurysms were previously ruptured (coiling=11 and clipping=3). The majority of aneurysms were located in the anterior circulation (87.5%) (figure 1).
Flow diagram summarizing patient and aneurysm characteristics as well as procedural and clinical outcome at discharge and follow-up.
The time interval between initial aneurysm treatment and FD placement was available for 19 subjects and was 21 months on average (range 2–68 months, median 14 months). Four patients had been previously treated at a different institution and no clear timeline could be established. One subject was lost to follow-up and presented 16 years after the initial treatment with aneurysm recurrence requiring retreatment. Median preprocedural mRS score was 0. Patient and aneurysm characteristics are summarized in table 1.
Patient characteristics and outcome
Antiplatelet regimen
Patients received 75 mg of clopidogrel and 81 mg of aspirin daily p.o. for at least 5 days before the elective procedure and were tested for P2Y12 reaction units (PRU) values using a VerifyNow P2Y12 assay. (Fifty per cent platelet inhibition or PRU <200 with platelet aggregation assay was considered therapeutic.) Dual-antiplatelet therapy was continued for a minimum of 6 months. Aspirin was continued lifelong.
Interventional procedure
All patients were treated with a PED and all but one with a single device. This one patient required placement of three telescoping PEDs for appropriate aneurysm neck coverage and parent vessel reconstruction of a dysplastic cavernous internal carotid artery segment. Additional coiling in combination with PED was performed in two subjects. In one patient, additional placement of a Neuroform stent was required owing to foreshortening of the distal part of the PED with associated partial uncovering of the aneurysm neck. It was decided not to place a second PED in order to avoid double coverage of associated perforators.
Outcome and follow-up evaluation
All patients tolerated the procedure well without complications. Evaluation of the mRS score after the procedure showed no change from baseline in all patients.
On follow-up angiography, aneurysm occlusion was categorized as complete (100% by aneurysm volume), near-complete (aneurysm occlusion ≥90% by aneurysm volume), or partial (aneurysm occlusion <90% by aneurysm volume).
Six-month angiography follow-up was available for 18 aneurysms (11 ruptured and 7 unruptured, 75%) and showed complete or near-complete occlusion in 17 cases (94.4%). Follow-up mRS at 6-month was unchanged for all patients. The median mRS remained 0.
Follow-up angiography at 12 months was available in 15 subjects with 9 previously ruptured and 6 unruptured aneurysms. Overall, complete or near-complete occlusion was seen in 14 patients (93.3%). Neurologic follow-up at 12 months showed no change in mRS for all patients (figure 1).
IAs that were previously coiled showed complete or near-complete occlusion in 100% of cases at 6-and 12 months’ follow-up, respectively. Previously clipped IAs showed complete or near-complete IA occlusion in 80% of cases (4/5) at 6 months and 75% of cases (3/4) at 12-months’ follow-up. Details of treatment, clinical outcome, and follow-up angiography are summarized in table 1.
Ruptured aneurysms
Six-month follow-up was available for 11 patients with previously treated ruptured aneurysms. Nine patients showed complete occlusion (81.8%) and two showed near-complete occlusion (18.2%). Mild IH was seen in two cases and moderate IH in one. Interestingly, IH was only observed in cases of previously ruptured aneurysms.
Twelve-month follow-up was available for nine patients. Eight IAs showed a complete occlusion (88.9%) and one showed a near-complete occlusion (11.1%). Progression from near-complete occlusion at 6 months to complete occlusion at 12 months was seen in one case.
Previously moderate IH seen at 6 months had decreased to mild IH at 12 months. One patient demonstrated new mild IH, which was not present at the 6-month follow-up.
Unruptured aneurysms
Six-month follow-up was available for seven patients with previously treated unruptured aneurysms. Three cases each showed complete and near-complete occlusion (42.9% each) and one showed partial occlusion (14.2%). No IH was seen.
Twelve-month follow up was available for six patients. Two patients showed a complete occlusion (33%), three near-complete occlusion (50%), and one showed stable partial occlusion (17%). There was no change in the degree of aneurysm occlusion from 6 to 12-months’ follow-up. No IH was observed.
Comparison of aneurysm occlusion rates in ruptured and unruptured aneurysms at 6- and 12-month follow-up is shown in figures 2 and 3.
Right internal carotid artery angiogram shows a previously unruptured right posterior communicating artery aneurysm status after coiling with complete aneurysm occlusion (A). A follow-up angiogram shows coil compaction with recanalization of the aneurysm neck measuring approximately 3.2×4.5×3.0 mm (B, arrow). The patient underwent successful flow diverter placement (C), resulting in immediate inflow reduction shown by stagnation of contrast within the recanalized part of the aneurysm (D, arrow). Two-year angiography follow-up shows no residual aneurysm filling with complete vascular remodeling (E and F).
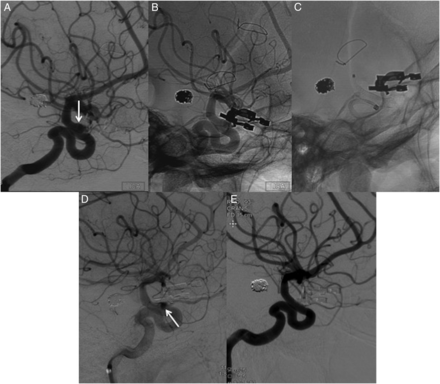
Left internal carotid artery angiogram shows recanalization of a previously clipped ruptured ophthalmic artery aneurysm (A arrow, B). The patient had prior coiling of a basilar tip aneurysm. The patient underwent successful placement of a flow diverter of the ophthalmic artery aneurysm (C). After placement of the flow diverter there is stagnation of contrast within the recurrent part of the aneurysm (D, arrow). Six-month angiography follow-up shows a complete occlusion of the aneurysm with no evidence of intimal hyperplasia (E).
Discussion
FD for initial management of IAs has been widely accepted for its high occlusion rates as well as low recurrence and re-treatment rates.
However, limited information is available on the use of a PED as second-line treatment for recurrent IAs or residuals after prior surgical clipping or coil embolization. Also, data are sparse on FD outcome of previously treated ruptured as compared with unruptured IAs.15–18
In our study, 94% of patients (17/18) with previously treated ruptured and unruptured aneurysms showed complete or near-complete occlusion at 6 months and 93% (14/15) at the 12-month follow-up. This compares favorably with the occlusion rates reported in the literature for naïve, previously untreated aneurysms managed with a PED2 ,19 ,20 and allows us to conclude that there is no difference in FD performance among naïve aneurysms and aneurysms for which coiling or clipping has previously failed.
Our results are also comparable to those of other small studies evaluating PED performance in previously treated aneurysms, which report near-complete to complete occlusion in 82.1–100%.15–18
In 2015, Daou et al 18 reported near-complete to complete occlusion in 86.7% of recurrent, previously coiled aneurysms that were managed with a PED. The authors included 17 ruptured aneurysms (17/32). We achieved near-complete to complete occlusion in 100% of our previously coiled aneurysm cases and can therefore confirm those authors' conclusion that the use of a PED in previously coiled aneurysms is safe and effective. At the same time, we also evaluated the presence of IH in this subgroup and found only two cases of mild IH at 6 months, one of which had resolved at the 12-months’ follow-up.
Benaissa et al 15 evaluated the use of flow diverters in recanalized (n=21) and multitreated (n=8) aneurysms (including 24 ruptured aneurysms) after initial endovascular treatment between 2010 and 2012 in a retrospective, multicenter analysis. The majority of the recanalized aneurysms had been previously treated with coil embolization (n=19) or stent-assisted coiling (SACE) (n=2). Multitreated aneurysms had been previously treated with endovascular coiling, SACE, or stenting alone. The authors saw adequate occlusion in 82.1% of cases (23/28) at a mean of 6.1±4.9 months’ follow-up. Worsening clinical status was seen in two patients and transient morbidity in three patients, related to occurrence of hemodynamic, hemorrhagic, and thromboembolic complications after use of flow diverters.
We were able to include 24 patients in our single-center analysis with, 12-months’ follow-up in 15/24 cases, and saw near-complete to complete occlusion in 93% of patients. We did not observe any treatment-related complications resulting in worsening mRS. Results from the single-center offer uniformity of treatment, which might explain the overall better results. Another factor, which might explain the better results in our series, is that none of the cases had previously been treated with SACE. Owing to improper wall apposition, a stent may impede the intimal growth over the PED and provide substrate for an endoleak.
In addition, we were interested in comparing aneurysm occlusion rates, patient outcome, and presence of IH between pretreated ruptured and unruptured IAs. After flow diverter placement, previously ruptured aneurysms showed a higher occlusion rate than previously unruptured aneurysms. We saw no procedure-related complications and there was no change in mRS from baseline in either group.
Interestingly, the three cases of mild IH were seen only in patients with previously ruptured aneurysms. We did not observe a correlation between occurrence of IH and patient vascular risk factors or time from initial aneurysm treatment to flow diverter placement in these patients. Nevertheless, this observation may suggest a trend that previously ruptured aneurysms are more prone to develop IH during the healing process after flow diverter placement.
Given these promising results, use of the PED appears to be effective and safe in both pretreated ruptured and unruptured aneurysms.
The limitations of our study mainly include its design (single-center, retrospective review), small size, and short-term follow-up. Nevertheless, we believe that the ability to demonstrate no difference in patient outcome and PED performance independent of prior aneurysm rupture status, will allow us to more confidently choose FD as a second-line treatment in cases of aneurysm recurrence. We also feel that these results can contribute to reassurance of patients undergoing aneurysm retreatment with FD technology.
Conclusion
Management of recurrent and residual IAs with FD technology is feasible and safe with occlusion rates comparable to untreated, naïve aneurysms managed with a PED.
We found that there is no difference in device performance and patient clinical outcome between pretreated, previously ruptured or unruptured aneurysms.
References
Footnotes
Contributors Study design: ALK, AKW, and ASP. Data acquisition: ALK, KdMR, JDL, AKW, DER, FM, MH, CB, JLH, and ASP. Literature research: ALK, KdMR, JDL, DER, FM, TT, and MH. Data analysis and interpretation: ALK, KdMR, MJG, AKW, and ASP. Manuscript preparation: ALK and ASP. Revision of manuscript for important intellectual content: KdMR, JDL, AKW, DER, FM, MJG, MH, CB, TT, and JLH. Approval of final version of manuscript: all authors.
Competing interests MJG is a consultant for Codman Neurovascular and Stryker Neurovascular; research grants: NIH, eV3/Covidien Neurovascular, Codman Neurovascular, Fraunhofer Institute, Wyss Institute, Philips Healthcare, Stryker Neurovascular, Silk Road, Lazarus-Effect. AKW is a consultant for Stryker Neurovascular; research grants: Philips Healthcare, Wyss Institute; speaker: Harvard Postgraduate Course, Miami Cardiovascular Institute. ASP is a consultant for Codman Neurovascular, Stryker Neurovascular, and Covidien; research grant from Stryker Neurovascular and Covidien; speaker: Miami Cardiovascular Institute.
Ethics approval Institutional review board at University of Massachusetts Medical School.
Provenance and peer review Not commissioned; externally peer reviewed.