Article Text
Abstract
The recent publication of the results of the international study on unruptured intracranial aneurysms highlighted a paradox: there do not seem to be enough unruptured aneurysms in the population to account for the observed incidence of subarachnoid haemorrhage. Some authors have suggested that the answer to this paradox is that most aneurysms that bleed do so shortly after formation. This would mean that the bulk of subarachnoid haemorrhages come from recently formed rather than long standing aneurysms. This paradox and proposed answer are examined. The available statistics on the incidence of subarachnoid haemorrhage, the prevalence of unruptured aneurysms, and the risk of bleeding from unruptured aneurysms are used to place a maximum on the time interval between aneurysm formation and rupture. For aneurysms less than 10 mm in diameter in persons with no history of subarachnoid haemorrhage, an estimate of less than 42 weeks was made. The null hypothesis that such aneurysms pose a constant risk with time is rejected with p <10−9. In larger aneurysms the risk seems to be constant with time.
- subarachnoid aneurysm rupture
Statistics from Altmetric.com
Data on the angiographic prevalence of unruptured aneurysms and the incidence of subarachnoid haemorrhage (SAH) have been available for some years. Most western series give figures around 10/100 000 people/year as the incidence of SAH and around 1% as the angiographic prevalence of unruptured aneurysms. From this followed the calculation that unruptured aneurysms have a risk of bleeding of (10/100 000)/(1%) which equals 1%/year. Several series on the follow up of unruptured aneurysms supported this calculation1-5 but there were indications that small aneurysms in persons with no history of SAH from another source pose very low risks.6-8 The International Study of Unruptured Intracranial Aneurysms (ISUIA)9confirmed this result. Aneurysms under 10 mm in diameter in people with no history of SAH from another source (henceforth referred to as safe group aneurysms) had an annual risk of rupture of less than 0.05% in the ISUIA. The actual value was 0.028% and was based on only one observed bleed. There is a discrepancy of a factor of more than 20 from the rate calculated above. Several answers to this paradox are possible:
(1) One answer is that the low bleed risk refers only to safe group aneurysm and these do not account for all SAHs. Most SAHs may come from more dangerous aneurysms. This is not supported by other findings suggesting that 71% of aneurysmal SAHs are from safe group aneurysms.10 We examine presenting SAH and incidental aneurysms involving safe group aneurysms in isolation and the paradox remains largely unchanged.
(2) There may be an inadvertently introduced bias in the data used. Although this is possible it seems unlikely that any single error could be so dramatic and equally unlikely that all data could be in error to a lesser degree but all conspiring to push the discrepancy in the same direction.
(3) The data may be drawn from different populations. To explain the paradox would require one population with a high incidence of SAH and prevalence of aneurysms and another with a low incidence and prevalence and the incidence to be taken from the high one and the prevalence from the low one. Certain populations do seem to have a high incidence and prevalence (Finland, Japan) and others have low figures (Saudi Arabia, India).11 12 The data on which this paradox is based do not include statistics from such populations. Moreover, there is a considerable overlap between the populations from which the three statistics were drawn, all of them being largely North American or Northern European extraction so this seems an unlikely explanation.
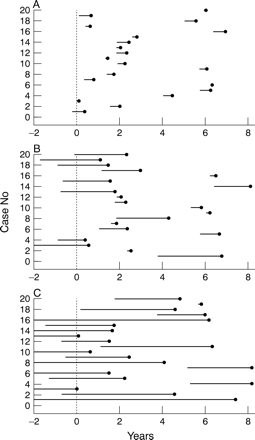
(4) The explanation we find most credible was suggested by Wieberset al 6 to explain this and a different but related paradox: most SAHs come from small aneurysms but most unruptured aneurysms which bleed under follow up are large. Wiebers et al suggested that most aneurysms that bleed do so shortly after formation and so are not detected as unruptured aneurysms. The paradox can be explained by postulating that aneurysms may form in people of any age and go through a comparatively brief high risk period immediately after formation. Those that do not bleed in this high risk period are, or become safer, posing a drastically reduced risk of further haemorrhage after an interval. In this scenario length bias ensures that most aneurysms detected incidentally have passed into the safe period giving a low bleed rate on follow up whereas most presenting with SAH are in the dangerous period. If this is the true explanation of the paradox then the statistics can be used to place a maximum on the duration of the early high risk period. Figure 1 is included to illustrate the principle involved. It does not form part of the calculations.
Illustration of the relation between the duration of an aneurysm's high risk period and the chance of detecting an aneurysm now in someone destined to have an SAH in the next 8.3 years. The figures are simulations of the occurrence of 20 SAHs over an 8.3 year period in a population screened for aneurysms with angiography at year 0 (the dotted lines indicate the time of screening). The high risk period of the aneurysms has a duration of 30 weeks in (A), 3 years in (B), and 10 years in (C). The risk is assumed to be 0 after this. The black dots indicate an SAH, the lines before them indicate the life span of the aneurysms causing the SAH. The number of these lines crossing the dotted screening line is an indication of how many aneurysms which subsequently bleed would be detected (=Π).
Methods
DATA EXTRACTION
The data items used are the haemorrhage rate for aneurysms under follow up from the ISUIA, the incidence of SAH, and the prevalence of aneurysms. We required that all statistics used should refer to similar populations. As the ISUIA data referred to the populations of North America and northern Europe, other studies on these populations were used. Medline searches were used to recover as many reports as possible. The reference lists of these reports were used to find others. Reports were chosen from this total if the populations studied were similar to the ISUIA population and if bias had been minimised.
PREVALENCE OF ANEURYSMS
In this study the term prevalence is used to refer to the prevalence of people harbouring aneurysms. People with multiple aneurysms were counted only once and not once for each of their aneurysms. As all unruptured aneurysms in the ISUIA were detected or at least confirmed on angiography it is the angiographic prevalence that is relevant. Postmortem data on the prevalence of aneurysms was not used directly. The angiographic prevalence of aneurysms is a difficult statistic for the present purpose. It is important that an estimate of the prevalence of aneurysms in the general population is used and serious bias is introduced by associations between the indications for angiography and aneurysms in most studies. Furthermore, many series include limited angiograms so presumably underestimate the prevalence of aneurysms. Two recent reviews on the angiographic prevalence of aneurysms are of value.13 14
Studies were included on the basis of the following criteria: studies had to cover a similar population to that covered by the ISUIA and studies had to be as free as possible from bias by associations between the indications for angiography and aneurysms.
Only one study could be found which fulfilled these criteria (Atkinsonet al 15). This was on a USA population. All the angiograms were the same (bilateral anterior circulation only) and every effort was made to eliminate biasing by associations between aneurysms and the indication for the angiograms. The scale of the problem of such bias can be judged from the experience of Atkinson et al. Of 9295 angiograms performed over a 7 year period only 278 were thought suitable for inclusion in his study. The result was a prevalence of 1.1%. The age distribution of the study population was unlike that of the population as a whole. In particular, persons under 30 years of age were underrepresented by a factor of more than 7. This could lead to significant bias as it is suspected that the prevalence of aneurysms in this age group is lower than in the rest of the population. The solution we have adopted to this problem is to truncate the series of Atkinson et al to only those subjects aged 30 years or more. The incidence data are truncated in a similar manner so that the age distributions of the populations under consideration are comparable. After this truncation the result was that 1.2% of those over 30 years old have unruptured aneurysms. Postmortem data show 94%-98% of aneurysms, to be in the anterior circulation16 and this was used to adjust the result of Atkinson et al to include aneurysms of the posterior circulation as no comparable angiographic data could be found. All three of the aneurysms found by Atkinsonet al were under 10 mm in diameter so no adjustment was made for small aneurysms. The final prevalence of safe group aneurysms among people 30 years or more was 1.25%.
INCIDENCE OF SAH
The world literature contains many reports on the incidence of SAH and these range from 1 to 96/100 000 people/year. On the whole Japan and Finland have shown high incidences while India and the Middle East have shown low incidences. Three recent reviews have clarified the statistics.11 16 17 Reports from the United States were chosen because of the population overlap with the series of Atkinsonet al and the ISUIA. We have identified eight such reports. Seven of these18-24 give incidences of SAH ranging from 6 to 16/100 000 people/year. One outlier was the Framingham study25 which gave an incidence of 28/100 000 people/year. This was not based on a total population but on a population entered into the study at the age of 30 or older and followed up for 26 years. The high incidence reflects the fact that the denominator excluded younger people who have a much lower incidence of SAH and who are included in other studies. Because of the age distribution of the study of Atkinson et alwe have restricted our calculations to adults only and have truncated these series to exclude younger persons. The age range differs from one study to another depending on the age bands reported. The age ranges were 35 or older,18 22 23 26 30 or older,24 25 25 or older,19 and 20 or older20. Most of the studies reported the incidence as SAHs/100 000 total population/year. We adjusted the denominator to exclude younger people by referring to United States population pyramids.27 For example Longstreth et al 19 found 171 SAHs in 1800 000 patient-years of observation to give an incidence of 9.4/100 000 people/year. Of these SAHs 169 were in people more than 25 years old. Reference to the 1988 population pyramid for the United States shows that 64% of the population was 25 years or older in 1988 so our adjusted incidence was 169/(64% of 100 000) which came to 14.5 SAHs/100 000 people ⩾25 years old/year. Data from these eight studies were expressed as rates of SAH/100 000 people over the relevant age (henceforth referred to as adults)/year. Among the eight studies a total of 516 SAHs were found in an estimated 3 000 000 adult-years of observation giving an incidence of 17.1 SAHs/100 000 adults/year. Further studies report that about 80% of spontaneous SAHs are from aneurysms.28 29Correcting for aneurysmal bleeds only gives 13.7 bleeds/100 000 adults/year in the United States. Subarachnoid haemorrhagess can be divided into the same groups as used in the ISUIA (those with aneurysms under 10 mm in diameter with no history of SAH, those under 10 mm in diameter with a history of SAH, and those of 10 mm or more in diameter). Under this division most SAHs come from aneurysms which would be in the safe group if they were unruptured. In the cooperative study,10 71% of SAHs were from aneurysms in the safe group, the median diameter being 7 mm. Freytag30 found the expanded class of “safe group aneurysms plus those of 10 mm in diameter” (safe group aneurysms are under10 mm) accounted for 80% of aneurysmal SAHs at necropsy. The cooperative figure was used to estimate the incidence of SAH from safe group aneurysms to be 9.7/100 000 adults/year.
CALCULATIONS
The following symbols are used:
i=The observed incidence of SAH from safe group aneurysms (9.735/100000 adult years).
p=The observed prevalence of safe group aneurysms (1.25%).
r=The rate of SAH from safe group aneurysms (0.028%/year from the ISUIA).
R(x)=The risk of an aneurysm rupturing/yearx years after it has formed.
Rl(x)=A special case ofR(x) where R=a constant for x ⩽l and R=0 for x > l.
Rz(x)=A special case ofR(x) where R=a constant for x ⩽z and R=0.00028/year for
x > z.
ΠR=The probability of detecting an aneurysm now in someone destined to have an SAH from a safe group aneurysm in the next 8.3 years as calculated by integrating R(x).
ΠD= The probability of detecting an aneurysm now in someone destined to suffer a SAH from a safe group aneurysm in the next 8.3 years as calculated from observed statistics.
ΦR=The total probability that an aneurysm will rupture at some time as calculated by integrating R(x).
ΦD.= The total probability that an aneurysm will rupture at some time as calculated from observed statistics.
Qf(t)=The probability/year that an aneurysm found now was formed t years ago.
P(B‖D)=The probability of a bleed in the coming 8.3 years in someone in whom an aneurysm is detected now.
a=The current age of a patient with an unruptured aneurysm - taken as Atkinson's mean age of 53 years.
k=A value such thatR(>k) is negligible for calculating ΦR.
j=Probability distribution function of

The aim is to take any arbitrary probability density function of time (which could represent aneurysm bleed risk/year) and to assess it for compatibility with the observed data. Such functions are referred to as R(x) whereR is the probability/year of SAH andx is time from aneurysmformation. The technique involves two probabilities:
The probability of detecting an aneurysm now in someone destined to have an SAH from a safe group aneurysm in the next 8.3 years is called Π.
The total probability that an aneurysm will rupture at some time is called Φ.
Both Φ and Π can be calculated for R(x)by integration in which case they are called ΠR and ΦR. Φ And Π can also be estimated for the true risk profile of aneurysms by calculations from observed statistical data and these estimates are called ΠD and ΦD. ForR(x) to be consistent with observed data requires that:
ΠR=ΠD (1)
and ΦR=ΦD (2)
The basis of the method is in solving these two simultaneous equations.
ΠD
ΠD is the probability of detecting an aneurysm now in someone destined to have an SAH in the next 8.3 years. The time was chosen because it is the mean ISUIA follow up time. ΠD Is calculated by estimating the number of SAHs in the screened population from which the ISUIA safe group was drawn and dividing the number of SAHs that occurred in the ISUIA safe group by this estimate.
In the ISUIA 424 patients with safe group aneurysms were followed up for a mean of 8.3 years. Using the prevalence of unruptured safe group aneurysms (1.25%) we can estimate that 424/0.0125 or about 34 000 patients were angiogrammed in the population from which the data was drawn. Using the incidence of SAH from safe group aneurysms in this population (9.7/100 000 patient-years) it can be calculated that that 34 000×8.3×9.7/100 000 or around 27 SAHs would be expected in this population in 8.3 years. Only 1 SAH was found (42–48 months after the angiogram) in the ISUIA safe group. Observation thus gave a value of 1/27 for ΠD. Algebraically this is expressed:
ΠD=rp/i (3)
ΦD
ΦD Is the proportion of aneurysms that eventually rupture. It was estimated by dividing the annual incidence of SAH by the annual incidence of aneurysm formation. For this estimate the assumption was made that once an aneurysm forms it persists for life and that the proportion that disappear is negligible. The age specific prevalence of unruptured aneurysms can be differentiated with respect to age to get an age specific incidence of unruptured aneurysm formation. If our conclusions are correct this is not the same population as those aneurysms that do rupture so the incidence of SAH is added to this figure to get the overall incidence of aneurysm formation. This leads to a rather uncertain estimate of the incidence of aneurysm formation because our assumption that aneurysms do not disappear may be incorrect and age specific prevalence data are sparse and unreliable. It turns out, however, that the final result is relatively insensitive to the value of this estimate. DuBoulay's angiographic study31 gives age stratification of aneurysm prevalence and suggests a linear increase in prevalence with age from infancy to middle age but patient selection was marked. Meyeret al
32 reported 23 patients under 18 years of age with intracranial aneurysms but the size of the referring population is difficult to estimate. Postmortem studies have generally not found aneurysms in children although populations are small. Our present results argue strongly against a substantial proportion of aneurysms arising in childhood. The crude approximation we use is to assume a constant rate of aneurysm formation from the age of 10 to the mean age of the population of Atkinsonet al of 53 years such that prevalence of Atkinson et al develops over this time. This gives the probability of forming an aneurysm as a constanti+p/(53–10)/year. ΦD is the proportion of aneurysms that bleed, which is the same as the probability of an aneurysm bleeding at some point once formed. This can be estimated by dividing the SAH rate in a population by the rate of aneurysm formation, so:

About one quarter of safe group aneurysms seem to bleed at some point.
The calculation of ΦR and ΠR is described in the .
Results
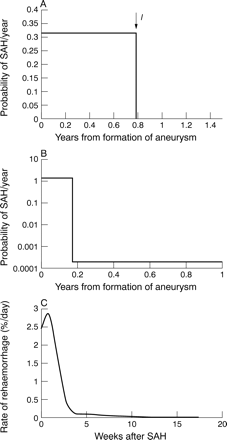
The simplest function for R(x) studied was a constant risk with time as calculated by dividing the incidence of SAH (i) by the prevalence of aneurysms (p). This gave a value of ΠR=1/1.41 or 0.71, which is far higher than that calculated from the statistics (1/27) and indicates incompatibility with observation. As expected if the ISUIA bleed rate was used as a constant rate, ΠR was 1/27 but the predicted incidence of SAH was only 0.5/100 000 person-years as opposed to the observed value of 9.7. This is just another way of stating the paradox. The simplestR(x) consistent with all the data has a constant risk falling to 0 suddenly at time l(Rl(x)) as shown in fig 2A. In this case ΠR was found to be linearly related tol according to ΠR≅0.06l giving ΠR=1/27 for l of about 41 weeks with a 95% confidence interval of 0—83 weeks. Of all theR(x)s consistent with observation this one has the longest period of high risk. If any ongoing risk beyondl is included inR(x), the duration of the early high risk period falls progressively with increasing ongoing risk, approaching a limit of 0 as ongoing risk increases towards the ISUIA value of 0.028%/year.
Graphs of various risk profiles R(x), which are consistent with observations. (A) is of Rl(x) with the risk falling to 0 at l=0.79 years (41 weeks). (B) R(x) has a small ongoing risk of 0.02%/year and a high risk period of 9 weeks. A logarithmic scale is used for the y axis. (C) is reproduced from the cooperative aneurysm study33 and is the observed post-SAH rehaemorrhage rate.
It can be seen that the ISUIA observed bleed which occurred after more than 182 weeks does not seem to be from an aneurysm in its early high risk period. We can therefore use the ISUIA value of 0.028%/year as an ongoing risk. The model Rl(x)can thus be refined to Rz(x)(fig 2 B) where the risk is constant until z when it falls to 0.028%/year. With no observed bleeds now in the high risk period a point estimate of the high risk period duration cannot be made but the 95% confidence interval can be reduced to 0–42 weeks.
Among the infinite number of possible compatibleR(x)s there is one other of particular interest. That is the post-SAH rehaemorrhage rate as measured in the cooperative study33 reproduced in fig 2 C. The ongoing risk is uncertain beyond 1 year but if this is set at 0% then Π is 0.01 (1/97). If it is set at 0.023% then Π is 0.0367 (1/27) and if it is set at 0.028% then Π is 0.06 (1/17). Φ For thisR(x) was 0.41. The rehaemorrhage risk profile thus seems entirely consistent with observed data on the risk profile of unruptured aneurysms.
The situation with larger aneurysms is simpler in that ΠD≅0.6 which is close to the value of 0.727 expected of aneurysms with a constant bleed risk. Large aneurysms thus behave as though they had a constant risk of haemorrhage with time.
These calculations cannot be applied to small aneurysms in persons with a history of SAH as we do not know the prevalence of this condition or the incidence of SAH after treatment for a ruptured aneurysm.
TREATMENT OF CONFIDENCE
Two measures of confidence are given. Firstly the statement “the risk of a safe group aneurysm rupturing does not depend on how long it has existed” is taken as a null hypothesis and tested. A distribution is calculated for the number of SAHs to occur in the total population given an angiogram (from which the ISUIA safe group was drawn) over 8.3 years. This distribution is based on a combination of the distributions of i and p. Further details are given in the . Using this distribution it was calculated that the probability of making the ISUIA observation of detecting one aneurysm which later bled (the p value) is<10−9 if the null hypothesis is true.
Secondly, a more elaborate analysis allows confidence limits to be applied to the duration of the high risk period. Of the risk profiles consistent with observationRl(x) has the longest high risk period. If any risk after time l is allowed the duration of the high risk period must fall to keepR(x) consistent with observation. As a long term risk of only 0.028%/year would indicate a very short duration the lower confidence limit for the duration of the high risk period is 0. A 95% upper confidence limit is calculated giving a 95% interval of 0–83 weeks. The confidence range is wide because bothp and r are based on few observations (3 and 1 respectively).i Is based on 516 observations and so adds little to the width of the 95% confidence interval.
Discussion
Our estimate is of a maximum ofunder 41 weeks (95% range 0–42 weeks). It should be emphasised that the functionsRl(x) andRz(x) are convenient from a mathematical point of view but neither can be the true risk profile. Consequently l andz are not estimates of the period of high risk but maximum values. A risk profile with a high risk period of 1 second would be just as consistent with observations as one with a high risk period of 41 weeks. No bleeds were found within the high risk period in the ISUIA. Hence the statistics give no clue about the minimum duration of the high risk period. Note also that the ISUIA safe group bleed rate of 0.028% or alternatively 28/100 000 adults/year is not significantly different from the general population rate of 0.017% or 17/100 000 adults/year. How confident can we be that the observed SAH came from the observed aneurysm and not a new one? If not, the risk posed by such aneurysms could be even lower than 0.028%/year, and the suggested maximum duration of the high risk period shorter.
Can anything be said about a lower limit of the high risk period? Although the statistics we have used give no indication of the minimum period of risk certain unconnected observations may do so. Commonly, when someone presents with SAH, in retrospect a history of headache within the last 1–8 weeks can be elicited.34 35 Such headaches may be due to earlier minor haemorrhages or be associated with aneurysm formation. In either case they suggest that the aneurysm/SAH process dates back at least 1–8 weeks before the presenting SAH in those cases with a harbinger headache.
A further clue may come from observations of the rebleed rate immediately after SAH. We suspect that the risk of an aneurysm bleeding either increases or remains the same immediately after a SAH. If this suspicion is correct the daily risk in the high risk period should be less than or equal to the immediate post-SAH rebleed rate which has been seen to be as high as 17%/day.36 Such a figure would suggest a minimum of around a day for the duration of the high risk period.
We think that a period of a few days to a few weeks is the most likely because it fits with the time distribution of pre-SAH headaches and with observations of the post-SAH rehaemorrhage rates.
These calculations cannot be applied to the group of small aneurysms in persons who have had an SAH from another aneurysm in the past but the results raise a question in this group. The observed haemorrhage rate may be from newly formed aneurysms in the acute phase rather than from the aneurysms detected angiographically. A simple analysis would suggest that as they have at least two aneurysms they must have developed them at the rate of at least 2/43 years if our simplified model is used. If they go on developing aneurysms at this rate, and our estimate that 25% of aneurysms bleed is correct, then the group would show a bleed rate of 0.25×2/43 or 1.2%/year. The bleed rate for this group was 0.5%. Even allowing for regression of aneurysm formation rates in individual subjects to a lower mean, many of these could be accounted for by new aneurysm formation. To be sure that these observed bleeds were not from newly formed aneurysms rather than the ones detected at angiography it would be necessary to have angiographic or postmortem data after the SAH in those patients who bled under follow up in the ISUIA study. Such information would be useful as it would have a bearing on the decision whether or not to treat aneurysms in this group.
The model of aneurysmal haemorrhage timing involving an early high risk period refers to the behaviour of groups of people harbouring aneurysms and not necessarily to individual aneurysms. One possible mechanism behind such behaviour is that individual aneurysms go through some healing process after formation and thus strengthen after an initial period of fragility. There is no convincing proof of such a process as yet. The other possibility is that aneurysms do have a constant risk of bleeding over time and an apparent early high risk period is the result of the selection of strong aneurysms to survive, the weak ones all bleeding within a few weeks of formation. The statistics we have used do not allow us to distinguish between these two possibilities. Deciding between these mechanisms will remain a matter of speculation until scientific proof arrives.
The results of these calculations stand or fall on the accuracy of the statistical measurements used. The confidence calculations give an indication of the degree of error expected due to random variation but not bias. We have attempted to minimise the amount of bias in the data used but one possible source merits further comment. If our assumptions about the behaviour of aneurysms are correct then an increasing prevalence with increasing age would be expected. We have attempted to allow for this by restricting our calculations to the adult population. None the less if, for example, the data on the prevalence of unruptured aneurysms were from a substantially younger population than the data on the incidence of SAH then the prevalence figure used could be inappropriately low. Of the studies on incidence we have used, not all give the mean age of patients but those that do18-20 23 24 are all in the range 55–58 years. The prevalence data we used were based on a population with a mean age of 53 years and we consider the discrepancy to be tolerably small.
Conclusions
Cerebral aneurysms seem to go through a period with a high risk of rupture immediately after formation suggesting that most acute SAHs are from recently formed aneurysms. After this initial period the risk of rupture falls to a low level. The duration of this high risk period may be between a day or two and around 8 weeks. We can say with greater confidence that it is less than 41 weeks (95% confidence interval 0–83 weeks). The probability of making the ISUIA observation if safe group aneurysms pose a constant risk with time is calculated as p<10−9. Larger aneurysms do seem to have a constant risk of rupture.
Mathematical method
ΠR
The first step towards calculating ΠR is to integrate R(x) forx from 0 to 8.3 years:

This gives the probability of SAH in the 8.3 year period which starts with the formation of the aneurysm. The next step is to calculate the probability of rupture in the 8.3 years afterdetection of the aneurysm. For thisR(x) is integrated from the age of the aneurysm when it is detected (t) tot+8.3 years. The value oft is of course unknown.t is treated as a random variable that can take values between 0 and the current age of the patient (a). The distribution used for t was derived from the incidence of the formation of aneurysms as discussed above.Qf(t) is used whereQf is the probability/year that an aneurysm found now was formed t years ago. The function used for Qf(t)is
Qf(t)=0 for(a-t) < 10 years and
Qf(t)=1/(a-10)for t=10 to ayears.
In other words aneurysms do not arise under the age of 10 years and arise at a constant rate thereafter. The probability of a bleed in the coming 8.3 years given that an aneurysm is detected now is called P(B‖D). It is calculated by taking the integral above, multiplying it by Qf(t) and integrating again for t between 0 and the patient's current age a for which we use Atkinson's mean age at diagnosis of 53 years:

According to the prevalence data, the probability of detecting a small aneurysm now in a person is p (1.25%) and according to the incidence data the probability of any person having an SAH in the next 8.3 years 8.3i. We now have three dependent probabilities: the probability of an aneurysm rupturing in the next 8.3 years if detected now or P(B‖D), the probability of detecting an aneurysm in a member of the general population nowp, and the probability of a member of the general population having a SAH in the next 8.3 years, 8.3i. These allow the use of Bayes' theorem to find the probability of detecting an aneurysm given that a bleed will occur in the next 8.3 years (ΠR).

ΦR
ΦR Is the probability that an aneurysm will eventually rupture once it has formed. It is calculated by integratingR(x) over the lifespan of the aneurysmk. k Is not known but allR(x) for which equations 1 and 2 hold must be negligible for x >10 years so 10 years is used for k.

TREATMENT OF CONFIDENCE
Two measures of confidence are given. Firstly, the statement “the risk of an aneurysm rupturing is constant with time” is taken as a null hypothesis and tested. p Andi are assumed to follow Poisson distributions and a compound distribution is calculated fori×8.3×424/p. This is the distribution of the number of SAHs to occur in the population given angiograms (mean population size 34 000) over 8.3 years. From this distribution it is calculated that the probability of making the ISUIA observation of detecting one zero safe group aneurysm which later bled is <10−9 or 1 in >1000 000 000.
Secondly, a more elaborate analysis allows confidence limits to be applied to the duration of the high risk period.Rl(x) Has the longest high risk period of R(x)s for any given value of ΠR, the duration of this periodl can be used as a guide to the upper limit of the duration of the high risk period. If any ongoing risk is allowed the duration of the high risk period must fall. To obtain an upper confidence limit i, p, andr can all be treated as random variables with Poisson probability distribution functionsf(i), g(p), h(r)respectively. Equations (3), (4), (5), and (6) are substituted into the simultaneous pair (1) and (2) to form the simultaneous pair (7) and (8):


Dividing (7) by (8) gives:
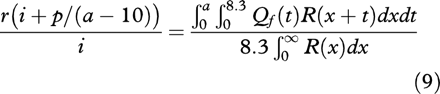
The observed statistics i, p, andr appear on the left hand side of equation(9), which can be solved for l ifRl(x) is used forR(x). A probability distribution for

is calculated. This we call
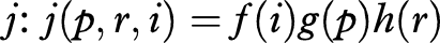
If this were calculated for all possible combinations ofi, p, and r then the resulting probabilities would sum to 1 but such a problem would be intractable on a PC. In practice the probabilities of making observations a long way from the mean is very remote and can be ignored with little loss of accuracy. The series were thus truncated so that all j(p,r,i )<10−9 were ignored. How close the sum of probabilities is to 1 is a useful check on the degree of error introduced by truncation. The truncation chosen produced:

The single upper 95% limit is at
r(i+p/(a-10))=0.0022. This was used as the left side of equation (9) which was then solved to give a value of 83 weeks for l so the 95% confidence interval is 0–83 weeks. The model is then improved toRz(x) with an ongoing risk of 0.028%/year and equation (9) solved to give a 95% confidence interval of 0–42 weeks for z.
In principle there are further improvements which could be made. Both the factors 0.71 (the proportion of aneurysmal SAHs to come from aneurysms <10 mm across) and 0.8 (the proportion of SAHs that are aneurysmal) could be treated as probability distributions rather than point estimates. In practice this would make the calculations intractable on the computers available to us for little improvement in accuracy as the bulk of the uncertainty resides in the measurementsp and r.